
For extraction of sodium from \[NaCl\] , the electrolytic mixture \[NaCl{\text{ }} + {\text{ }}N{a_3}Al{F_6} + {\text{ }}CaC{l_2}\] is used. During extraction process, only sodium is deposited on cathode but \[K\] and \[Ca\] do not because:
\[A.\,\,Na\,{\text{is more reactive than}}\,K\,{\text{and}}\,Ca\]
\[B.\,\,Na\,{\text{is less reactive than}}\,K\,{\text{and}}\,Ca\]
\[C.\,\,NaCl\,{\text{is less stable than }}N{a_3}Al{F_6}\,{\text{and}}\,CaC{l_2}\]
\[D.\,\,{\text{The discharge potential of }}N{a^ + }{\text{ is less than that is }}{K^ + }{\text{ and }}C{a^{2 + }}\]
Answer
552.3k+ views
Hint:This is the established instrument for the extraction of sodium from the electrolytic process. In this instrument there is having molten electrolyte and below that there will be molten aluminium. In the molten electrolyte \[N{a_3}Al{F_6} + {\text{ }}CaC{l_2}\] is used. In which, the calcium chloride is used for the increasing of electrical conductivity and \[N{a_3}Al{F_6}\] used for the decrease of the temperature of melting point. In this, we have carbon cathode and carbon anode.
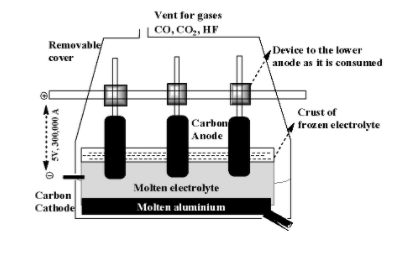
Complete step-by-step answer:In the question, we need to find while during the extraction process, only sodium is deposited on cathode but not potassium and calcium.
Let’s see the options and understand the reason behind it;
If we see option A which states that sodium is more reactive than potassium and calcium. If it is like this, it could react fast with any compound. So, option A is wrong.
Now, let’s see option B, which states sodium is less reactive than potassium and calcium. If it happens, the reaction would be occurring in the mixture itself. So, option B is wrong.
Now, let’s see the option C, which states that sodium chloride is less stable than \[N{a_3}Al{F_6}\,{\text{and }}CaC{l_2}\] . In this case, sodium chloride is the most stable compound. So, option C is wrong.
Now, let’s see the option D, which states that the discharge potential of sodium ion is less than the potassium ion and calcium ion. In this case, the sodium is having less reduction potential or discharge potential as compared to the potassium ion and calcium ion. Due to which the formation of the layer takes place at the cathode. Whereas, the potassium ion and calcium ions get exited fast as compared to sodium.
Note:There are several methods to extract sodium metal. One of the methods is Down’s process. The sodium is obtained from the electrolysis of molten sodium chloride. Potassium chloride and potassium fluoride are used to decrease the melting point of the mixture.

Complete step-by-step answer:In the question, we need to find while during the extraction process, only sodium is deposited on cathode but not potassium and calcium.
Let’s see the options and understand the reason behind it;
If we see option A which states that sodium is more reactive than potassium and calcium. If it is like this, it could react fast with any compound. So, option A is wrong.
Now, let’s see option B, which states sodium is less reactive than potassium and calcium. If it happens, the reaction would be occurring in the mixture itself. So, option B is wrong.
Now, let’s see the option C, which states that sodium chloride is less stable than \[N{a_3}Al{F_6}\,{\text{and }}CaC{l_2}\] . In this case, sodium chloride is the most stable compound. So, option C is wrong.
Now, let’s see the option D, which states that the discharge potential of sodium ion is less than the potassium ion and calcium ion. In this case, the sodium is having less reduction potential or discharge potential as compared to the potassium ion and calcium ion. Due to which the formation of the layer takes place at the cathode. Whereas, the potassium ion and calcium ions get exited fast as compared to sodium.
Note:There are several methods to extract sodium metal. One of the methods is Down’s process. The sodium is obtained from the electrolysis of molten sodium chloride. Potassium chloride and potassium fluoride are used to decrease the melting point of the mixture.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




