
For $ {{C}_{6}}{{H}_{5}}CHO\xrightarrow[\Delta ]{KCN(alc.)}A $ What is A?
(A) $ {{C}_{6}}{{H}_{5}}-CO-{{C}_{6}}{{H}_{5}} $
(B) $ {{C}_{6}}{{H}_{5}}-CHOH-CO-{{C}_{6}}{{H}_{5}} $
(C) $ {{C}_{6}}{{H}_{5}}-CHOH-{{C}_{6}}{{H}_{5}} $
(D) $ {{C}_{6}}{{H}_{5}}-CO-CO-{{C}_{6}}{{H}_{5}} $
Answer
487.8k+ views
Hint: Oxygen is more electronegative than carbon in the aldehyde functional group and thus carbon carries $ \delta + $ charge and oxygen carries $ \delta - $ charge. Due to this polarity, aldehyde undergoes a nucleophilic addition reaction. $ KCN $ is an ionic compound composed of potassium ion $ ({{K}^{+}}) $ and cyanide ion $ (C{{N}^{-}}) $ .
Complete answer:
$ KCN $ is an ionic compound composed of potassium ion $ ({{K}^{+}}) $ and cyanide ion $ (C{{N}^{-}}) $ . $ \Pi $ -electron cloud of the carbonyl group is asymmetrical because of greater electronegativity of oxygen in comparison to carbon. Therefore, the carbonyl group undergoes a nucleophilic addition reaction. The above reaction leads to the formation of benzoin which is illustrated in the following reaction.
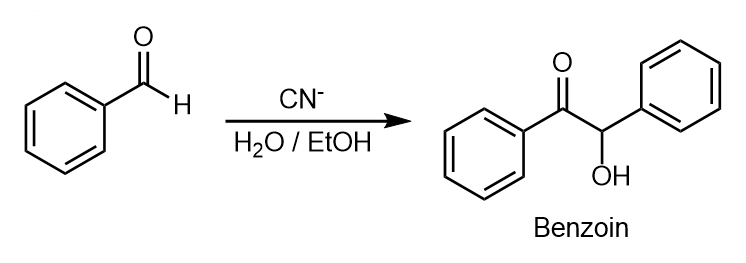
The above reaction occurs by the following mechanism.
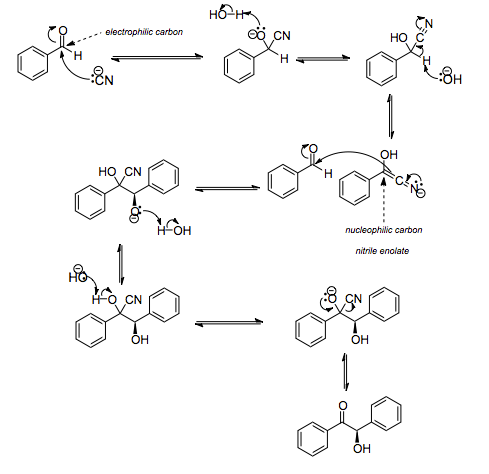
In the above reaction, cyanide ion $ (C{{N}^{-}}) $ acts as a nucleophile and thus attacks the carbonyl carbon to form tetrahedral intermediate cyanohydrin ion. Afterwards, nitrile enolate formation followed by nucleophilic addition and cyanide elimination leads to the formation of benzoin.
Hence, Benzoin is formed by the treatment of benzaldehyde $ ({{C}_{6}}{{H}_{5}}CHO) $ with $ KCN(alc.) $ .
Therefore, product A is benzoin, and option (B) is correct.
It is important to note that the treatment of benzaldehyde with $ KCN(alc.) $ leads to the formation of benzoin. Therefore, option (B) is correct. This reaction involves the sequence of nucleophilic addition reactions on carbonyl carbon to obtain the desired product.
Option (B) is correct.
Additional Information:
In contrast to the carbonyl functional group the $ \Pi $ -electron cloud of alkene is symmetrical and easily available for the attack of electrophile. Thus, alkene undergoes electrophilic addition reactions instead of nucleophilic addition reactions.
Note:
The compound formed in the above reaction is benzoin, which can be called an alpha-hydroxy carbonyl compound. This compound forms when a carbonyl compound is treated with a strong nucleophilic group. When a nucleophile like hydroxide is taken, it undergoes cannizzaro condensation and forms other products rather than benzoin.
Complete answer:
$ KCN $ is an ionic compound composed of potassium ion $ ({{K}^{+}}) $ and cyanide ion $ (C{{N}^{-}}) $ . $ \Pi $ -electron cloud of the carbonyl group is asymmetrical because of greater electronegativity of oxygen in comparison to carbon. Therefore, the carbonyl group undergoes a nucleophilic addition reaction. The above reaction leads to the formation of benzoin which is illustrated in the following reaction.

The above reaction occurs by the following mechanism.

In the above reaction, cyanide ion $ (C{{N}^{-}}) $ acts as a nucleophile and thus attacks the carbonyl carbon to form tetrahedral intermediate cyanohydrin ion. Afterwards, nitrile enolate formation followed by nucleophilic addition and cyanide elimination leads to the formation of benzoin.
Hence, Benzoin is formed by the treatment of benzaldehyde $ ({{C}_{6}}{{H}_{5}}CHO) $ with $ KCN(alc.) $ .
Therefore, product A is benzoin, and option (B) is correct.
It is important to note that the treatment of benzaldehyde with $ KCN(alc.) $ leads to the formation of benzoin. Therefore, option (B) is correct. This reaction involves the sequence of nucleophilic addition reactions on carbonyl carbon to obtain the desired product.
Option (B) is correct.
Additional Information:
In contrast to the carbonyl functional group the $ \Pi $ -electron cloud of alkene is symmetrical and easily available for the attack of electrophile. Thus, alkene undergoes electrophilic addition reactions instead of nucleophilic addition reactions.
Note:
The compound formed in the above reaction is benzoin, which can be called an alpha-hydroxy carbonyl compound. This compound forms when a carbonyl compound is treated with a strong nucleophilic group. When a nucleophile like hydroxide is taken, it undergoes cannizzaro condensation and forms other products rather than benzoin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




