
For an endothermic reaction where ${\bf{\Delta H}}$ represents the enthalpy of the reaction in ${\bf{kJ}}\;{\bf{mo}}{{\bf{l}}^{{\bf{ - 1}}}}$, the minimum value for the energy of activation will be:
A. less than $\Delta H$
B. zero
C. more than $\Delta H$
D. equal to $\Delta H$
Answer
592.2k+ views
Hint: We know that, endothermic reaction is the reaction in which heat is absorbed from the surroundings by the system that takes place to produce the product.
Complete step by step answer:
Activation energy is the minimum quantity of energy needed by a reactant to result in a chemical reaction. There are many units of measurement of activation energy such as kilojoule/mol, joule/mol etc.
The change of heat energy takes place when reactant changes to product are termed as enthalpy change. This enthalpy change is symbolised by $\Delta H$.
Now, we will draw the energy diagram for endothermic reaction.
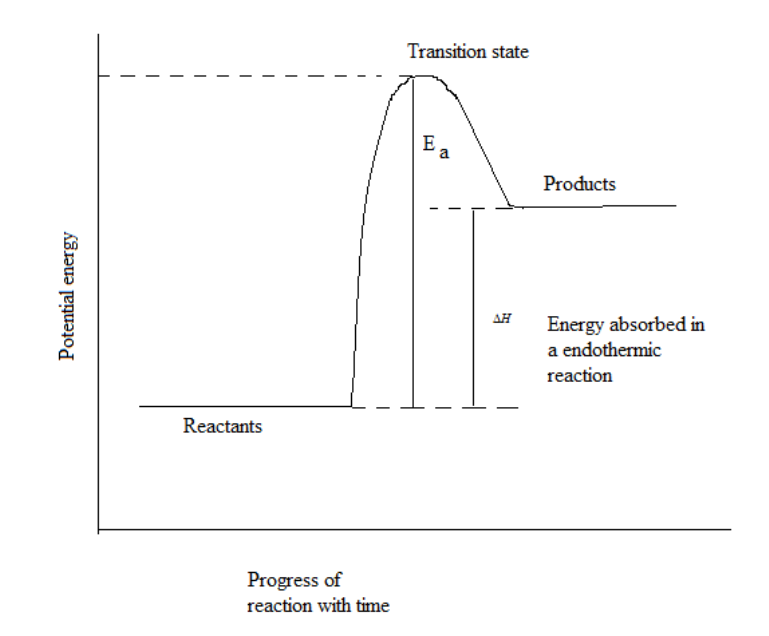
In endothermic reactions, the energy content of reactants is lower compared to energy of products as shown in the above energy diagram. Also we can say that stability of product is less than reactant. As the reaction proceeds in forward direction towards unstable entities, enthalpy for the reaction is positive in case of endothermic reaction.
Now, come to the question, as the minimum value of activation energy is the difference between energy of reactant and product. Also $\Delta H$ gives energy difference between product and reactant. From the above graph we can easily conclude that the minimum value of activation energy is equal to $\Delta H$.
Hence, option D is correct.
Note:
In exothermic reactions, the reactants possess greater energy than products. The reaction proceeds by releases of heat energy to the surroundings.
Complete step by step answer:
Activation energy is the minimum quantity of energy needed by a reactant to result in a chemical reaction. There are many units of measurement of activation energy such as kilojoule/mol, joule/mol etc.
The change of heat energy takes place when reactant changes to product are termed as enthalpy change. This enthalpy change is symbolised by $\Delta H$.
Now, we will draw the energy diagram for endothermic reaction.

In endothermic reactions, the energy content of reactants is lower compared to energy of products as shown in the above energy diagram. Also we can say that stability of product is less than reactant. As the reaction proceeds in forward direction towards unstable entities, enthalpy for the reaction is positive in case of endothermic reaction.
Now, come to the question, as the minimum value of activation energy is the difference between energy of reactant and product. Also $\Delta H$ gives energy difference between product and reactant. From the above graph we can easily conclude that the minimum value of activation energy is equal to $\Delta H$.
Hence, option D is correct.
Note:
In exothermic reactions, the reactants possess greater energy than products. The reaction proceeds by releases of heat energy to the surroundings.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




