
For a thermodynamic process to be reversible, the temperature difference between the body and the working substance should be
A) Zero
B) Minimum
C) Maximum
D) Infinity
Answer
575.1k+ views
Hint: We can see what are the conditions for a thermodynamic process to be reversible, then observe and conclude the relationship between the change in their temperatures to get the correct answer.
Complete step by step answer:
Some important definitions:
Thermodynamic process: ‘thermal’ or ‘thermo’ refers to heat and ‘dynamic’ means motion, so when the heat is transferred (moved) within the system or between the systems, the process is called thermodynamic process.
Reversible thermodynamic process: In a process when the system and surrounding return to their original state after undergoing reactions without any change in the universe is known as reversible process.
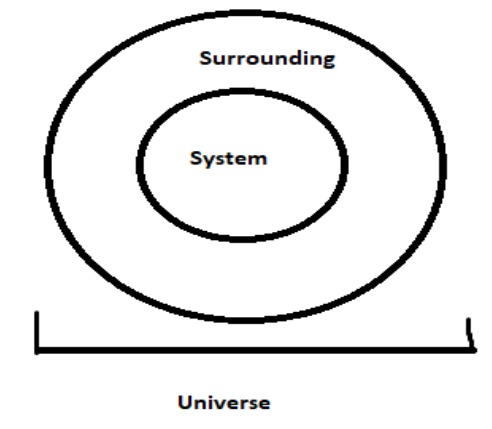
Entropy: Entropy is the randomness of the system. It is the loss of energy when the work in a system is done. For a reversible process, entropy is always zero because there is no change in energy between the systems and they return to their original state.
Therefore, it can be seen that for a thermodynamic process to be reversible, there should be no change in energy and entropy, for that, the body and the working substance should be at the same temperature providing no difference between their temperatures.
Thus, the difference between the body and the working substance should be Zero and the correct option is A).
So, the correct answer is “Option A”.
Note:
Different types of thermodynamic processes based on their conditions are:
Isothermal: Constant temperature (iso means same)
Adiabatic: No heat exchange
Isochoric: No change in system’s volume
Isobaric: Constant pressure
Remember that entropy can never decrease, either it will be constant or it will increase.
It remains constant for reversible reactions and increases for irreversible reactions.
Complete step by step answer:
Some important definitions:
Thermodynamic process: ‘thermal’ or ‘thermo’ refers to heat and ‘dynamic’ means motion, so when the heat is transferred (moved) within the system or between the systems, the process is called thermodynamic process.
Reversible thermodynamic process: In a process when the system and surrounding return to their original state after undergoing reactions without any change in the universe is known as reversible process.


Entropy: Entropy is the randomness of the system. It is the loss of energy when the work in a system is done. For a reversible process, entropy is always zero because there is no change in energy between the systems and they return to their original state.
Therefore, it can be seen that for a thermodynamic process to be reversible, there should be no change in energy and entropy, for that, the body and the working substance should be at the same temperature providing no difference between their temperatures.
Thus, the difference between the body and the working substance should be Zero and the correct option is A).
So, the correct answer is “Option A”.
Note:
Different types of thermodynamic processes based on their conditions are:
Isothermal: Constant temperature (iso means same)
Adiabatic: No heat exchange
Isochoric: No change in system’s volume
Isobaric: Constant pressure
Remember that entropy can never decrease, either it will be constant or it will increase.
It remains constant for reversible reactions and increases for irreversible reactions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




