
For $4$ -pentanoic acid when treated with ${I_2}$ and $NaHC{O_3}$ gives:
A. $4,5-diiodo pentanoic acid$
B. $5 -iodomethyl-dihydrofuran-2-one$
C. $5-iodo tetrahydropyran-2-one$
D. $4-pentenoyl iodide$
Answer
513.3k+ views
Hint: To solve this question try to recall Iodolactonization. Halolactonization is a reaction in which a ring (lactone) is formed by the addition of oxygen and iodine to the side of the carbon-carbon double bond. Halolactonization generally forms four to six-membered lactones. The strength of the reaction comprises the mild conditions of the versatile iodine atom into the product.
Complete answer:
When $4-pentanoic acid$ when treated with ${I_2}$ and $NaHC{O_3}$ , there is a formation of a positively charged Iodonium ion in the molecule that contains a carboxylic acid. The oxygen of the carboxyl acts as a nucleophile and attacks the Iodonium ion and forms a lactone ring.
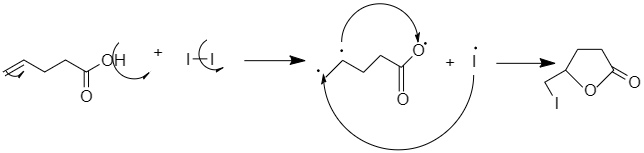
The reaction is performed under the basic condition to increase the nucleophilicity of the carboxyl group.
So the correct answer is option B.
$4-pentenoic acid$ when treated with ${I_2}$ and $NaHC{O_3}$ gives $5-iodomethyl-dihydrofuran-2-one$ .
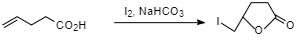
Additional Information: “lactone” name was coined by a French chemist Theophile-Jules Pelouze. The word lactone derives from the ring compound called lactide, formed from dehydration of $2-hydroxypropanoic acid. Lactones contribute to the flavor of fruit, unfermented and fermented dairy products.
Note:
Stereoselective Iodolactonization is used to synthesize many natural products including the medicinal application such as vernolepin and venomenon, used in tumor inhibition. Vibralactone is a pancreatic lipase inhibitor that is used in the treatment of obesity. Iodolactonization is also used to synthesize prostaglandins.
Bromolactonization is much less used because the electrophilic addition of bromine to alkene can compete with bromolactonization reaction and reduces the yield of the desired lactone.
Complete answer:
When $4-pentanoic acid$ when treated with ${I_2}$ and $NaHC{O_3}$ , there is a formation of a positively charged Iodonium ion in the molecule that contains a carboxylic acid. The oxygen of the carboxyl acts as a nucleophile and attacks the Iodonium ion and forms a lactone ring.

The reaction is performed under the basic condition to increase the nucleophilicity of the carboxyl group.
So the correct answer is option B.
$4-pentenoic acid$ when treated with ${I_2}$ and $NaHC{O_3}$ gives $5-iodomethyl-dihydrofuran-2-one$ .
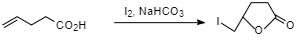
Additional Information: “lactone” name was coined by a French chemist Theophile-Jules Pelouze. The word lactone derives from the ring compound called lactide, formed from dehydration of $2-hydroxypropanoic acid. Lactones contribute to the flavor of fruit, unfermented and fermented dairy products.
Note:
Stereoselective Iodolactonization is used to synthesize many natural products including the medicinal application such as vernolepin and venomenon, used in tumor inhibition. Vibralactone is a pancreatic lipase inhibitor that is used in the treatment of obesity. Iodolactonization is also used to synthesize prostaglandins.
Bromolactonization is much less used because the electrophilic addition of bromine to alkene can compete with bromolactonization reaction and reduces the yield of the desired lactone.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

How was the Civil Disobedience Movement different from class 12 social science CBSE

How is democracy better than other forms of government class 12 social science CBSE




