
Following is an example of an $E2$ reaction
A.True
B.False

Answer
573.3k+ views
Hint: We know the elimination reaction is a kind of organic reaction where two substituents are removed from a molecule in a one- or two-step process. If the mechanism proceeds in one-step, then it is called as $E2$ reaction and if the reaction proceeds in two-step, then it is called as $E1$ reaction. $E2$ reaction is bimolecular reaction, whereas $E1$ reaction is unimolecular reaction.
Complete step by step solution:
We have to remember that an elimination reaction is the class of reactions mostly used for the conversion of saturated compounds to unsaturated compounds. The action of acids, bases, or metals results in the elimination of several groups of atoms or pairs of atoms from a molecule. It can also occur by the process of heating at high temperature.
We know that the $E2$ reaction is a bimolecular reaction and takes place in one step. In $E2$ elimination reaction the carbon-hydrogen and carbon-halogen bonds are broken, and a double bond is formed. In $E2$ reaction, a strong base is used such that it removes weakly acidic hydrogen.
We know that the $E1$ reaction is a unimolecular reaction and takes place in two steps. The two major steps in the $E1$ reaction are ionization and deprotonation. In the ionization step, the carbon-halogen bond breaks and intermediate carbocation is formed. In the second step, carbocation is deprotonated.
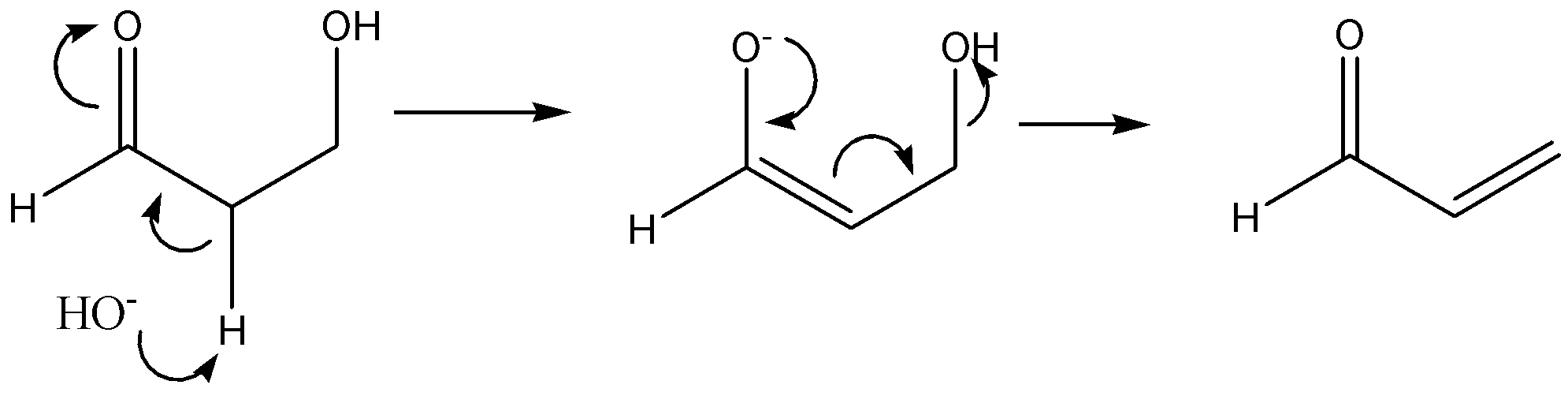
The given reaction is,
The above reaction is an acid-catalyzed dehydration of enol. The acid-catalysed dehydration of enol is an example of elimination reaction. It involves the removal of molecules of water (or) in the removal of a molecule of alcohol. In this reaction, $HOH$ substituent is removed that leads to the formation of a double bond. Thus, this reaction is an example of an $E1$ elimination reaction.
Therefore,option (B) is correct.
Note:
We have to remember that an $E1$ mechanism shares the characteristics of the ${{\text{S}}_{\text{N}}}{\text{1}}$ reaction. The 1st step in the E1 reaction is the formation of carbocation as an intermediate by the removal of the leaving group. Generally, this step takes a long time to complete and this is the rate-determining step.
Complete step by step solution:
We have to remember that an elimination reaction is the class of reactions mostly used for the conversion of saturated compounds to unsaturated compounds. The action of acids, bases, or metals results in the elimination of several groups of atoms or pairs of atoms from a molecule. It can also occur by the process of heating at high temperature.
We know that the $E2$ reaction is a bimolecular reaction and takes place in one step. In $E2$ elimination reaction the carbon-hydrogen and carbon-halogen bonds are broken, and a double bond is formed. In $E2$ reaction, a strong base is used such that it removes weakly acidic hydrogen.
We know that the $E1$ reaction is a unimolecular reaction and takes place in two steps. The two major steps in the $E1$ reaction are ionization and deprotonation. In the ionization step, the carbon-halogen bond breaks and intermediate carbocation is formed. In the second step, carbocation is deprotonated.
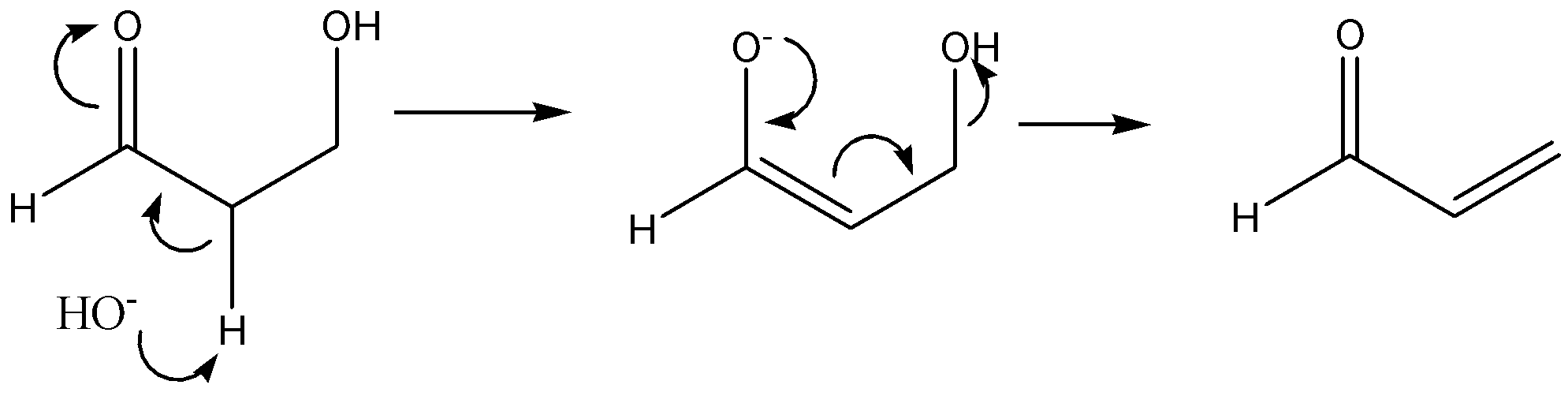
The given reaction is,

The above reaction is an acid-catalyzed dehydration of enol. The acid-catalysed dehydration of enol is an example of elimination reaction. It involves the removal of molecules of water (or) in the removal of a molecule of alcohol. In this reaction, $HOH$ substituent is removed that leads to the formation of a double bond. Thus, this reaction is an example of an $E1$ elimination reaction.
Therefore,option (B) is correct.
Note:
We have to remember that an $E1$ mechanism shares the characteristics of the ${{\text{S}}_{\text{N}}}{\text{1}}$ reaction. The 1st step in the E1 reaction is the formation of carbocation as an intermediate by the removal of the leaving group. Generally, this step takes a long time to complete and this is the rate-determining step.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




