
Find the product W.

1)
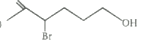 3)
3)

2)
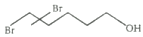 4)
4)
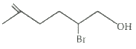





Answer
506.4k+ views
Hint: it is a type of addition followed by an elimination reaction. In this reaction, first, the bromine molecule will attack at the unsaturated site, i.e. the alkene region. This would be an anti-addition. Once the bromine molecule has attached to the unsaturated site, next the bromine atom and the hydrogen of the hydroxyl group will undergo the elimination reaction, thus forming an intramolecular elimination reaction’s product.
Complete answer:
First, we need to understand how the given reactants will react. $B{r_2}{\text{ and }}C{H_2}C{l_2}$ together act as an additional agent and add to the unsaturated compound. This is known as an additional reaction.
Hence, in the first, the reaction would take place as follows:
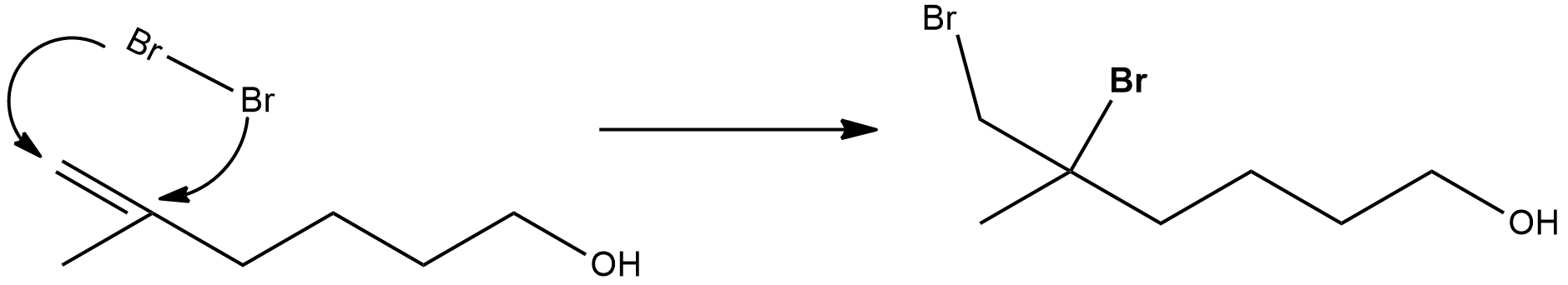
After this, the next step is the reaction is the intramolecular elimination of hydrogen bromide$HBr$.and undergoes ring formation. From here itself, it can be inferred that it will form a cyclic molecule and the reaction involved is given below:
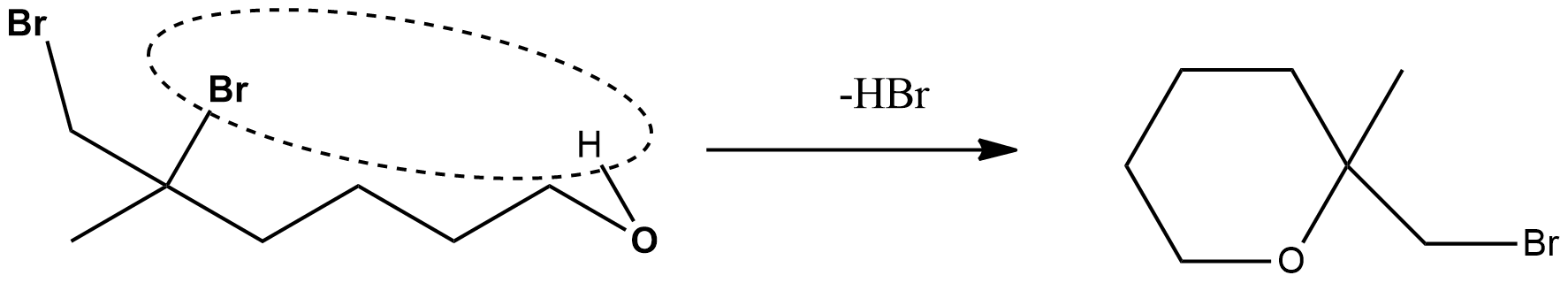
Thus, the final product will be an epoxide ring, which is given into option 3.
So, the correct answer is “Option 3”.
Note:
It should be noted that out of the two bromines, that bromine is eliminated in the form of hydrogen peroxide upon whose elimination, the ring that will be formed should be a stable one. It is a six membered ring that would be formed since it is the most stable form. Thereby, the product takes such form.
Complete answer:
First, we need to understand how the given reactants will react. $B{r_2}{\text{ and }}C{H_2}C{l_2}$ together act as an additional agent and add to the unsaturated compound. This is known as an additional reaction.
Hence, in the first, the reaction would take place as follows:

After this, the next step is the reaction is the intramolecular elimination of hydrogen bromide$HBr$.and undergoes ring formation. From here itself, it can be inferred that it will form a cyclic molecule and the reaction involved is given below:
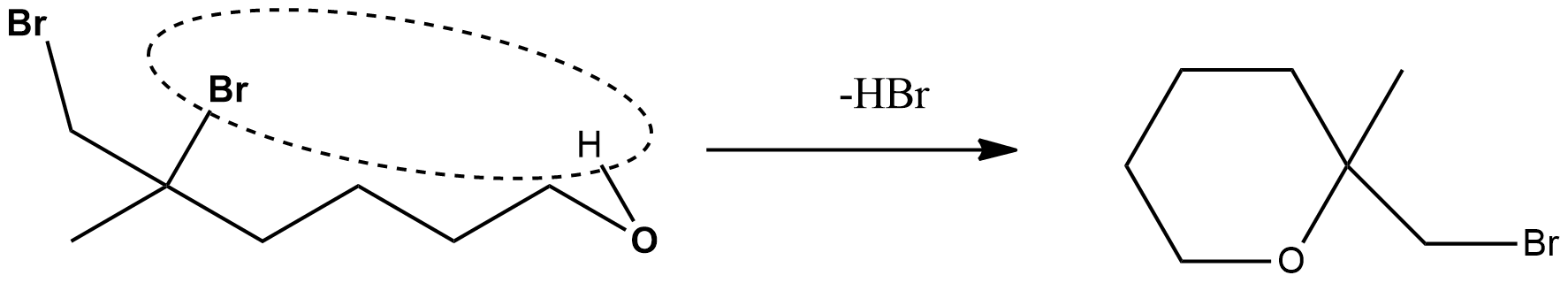
Thus, the final product will be an epoxide ring, which is given into option 3.
So, the correct answer is “Option 3”.
Note:
It should be noted that out of the two bromines, that bromine is eliminated in the form of hydrogen peroxide upon whose elimination, the ring that will be formed should be a stable one. It is a six membered ring that would be formed since it is the most stable form. Thereby, the product takes such form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




