
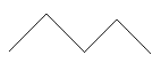
Find the number of mono chlorinated products with and without stereoisomers of the following compound.

Answer
506.4k+ views
Hint: The given compound is $ n - $ pentane. Chlorination is the process of substituting chlorine $ \left( {Cl} \right) $ in place of hydrogen. Here we have to perform mono chlorination, which means only one $ \left( {Cl} \right) $ will be substituted. Therefore we have to try it for different positions of chlorine.
Complete answer:
$ n - $ pentane has formula $ {C_5}{H_{12}} $ which means it is an alkane . Hence chlorination takes place in the presence of sunlight. Since we have to mono chlorination of the given pentane which means only a single chlorine atom will replace a hydrogen atom at a time. For this reaction can be written as:
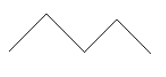
$ - $ $ \xrightarrow{{C{l_{2{\text{ }}}}/{\text{ hv}}}} $ Mono chlorinated products
Start from one side of the chain and number the carbon. Then write the IUPAC name of each product when we encounter with repeated name stop there. Therefore we will have products as:
$ \left( 1 \right) $
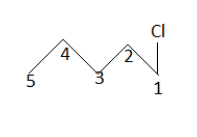
IUPAC name: $ 1 - $ chloropentane
$ \left( 2 \right) $
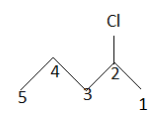
IUPAC name: $ 2 - $ chloropentane
$ \left( 3 \right) $
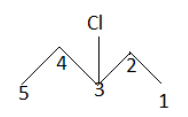
IUPAC name: $ 3 - $ chloropentane
After this the product which will be mono chlorinated will be named as $ 2 - $ chloropentane as the numbering of the carbon atom gets changed. So till now we get three mono chlorinated products. But if we look at the second product we notice another thing. The carbon on which chlorine gets attached now becomes a chiral carbon .Therefore it is an enantiomer having R and S configuration.
Therefore in total we have $ 4 $ mono chlorinated $ n - $ pentane. In this we have $ 2 - $ chloropentane which will act as stereoisomers.
Note:
Chiral carbon is that carbon which has all its four valencies different from each other. Chiral carbon is also an optical active compound. While naming the carbon chain do not make isomers of pentane like iso $ - $ pentane .We have to give chlorinated products of $ n - $ pentane only.
Complete answer:
$ n - $ pentane has formula $ {C_5}{H_{12}} $ which means it is an alkane . Hence chlorination takes place in the presence of sunlight. Since we have to mono chlorination of the given pentane which means only a single chlorine atom will replace a hydrogen atom at a time. For this reaction can be written as:

$ - $ $ \xrightarrow{{C{l_{2{\text{ }}}}/{\text{ hv}}}} $ Mono chlorinated products
Start from one side of the chain and number the carbon. Then write the IUPAC name of each product when we encounter with repeated name stop there. Therefore we will have products as:
$ \left( 1 \right) $

IUPAC name: $ 1 - $ chloropentane
$ \left( 2 \right) $

IUPAC name: $ 2 - $ chloropentane
$ \left( 3 \right) $

IUPAC name: $ 3 - $ chloropentane
After this the product which will be mono chlorinated will be named as $ 2 - $ chloropentane as the numbering of the carbon atom gets changed. So till now we get three mono chlorinated products. But if we look at the second product we notice another thing. The carbon on which chlorine gets attached now becomes a chiral carbon .Therefore it is an enantiomer having R and S configuration.
Therefore in total we have $ 4 $ mono chlorinated $ n - $ pentane. In this we have $ 2 - $ chloropentane which will act as stereoisomers.
Note:
Chiral carbon is that carbon which has all its four valencies different from each other. Chiral carbon is also an optical active compound. While naming the carbon chain do not make isomers of pentane like iso $ - $ pentane .We have to give chlorinated products of $ n - $ pentane only.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




