
Find the number of lone pairs of electrons on $Xe$ in $XeO{F_4}$.
Answer
580.2k+ views
Hint: To find the number of lone pairs of electrons on $Xe$, we need to draw the structure of $XeO{F_4}$. We should also be aware of the oxidation states in which the central atom $Xe$ can exist, so as to know the number of electrons involved in bonding and number of lone pairs.
Complete step by step answer:
Xenon is found in multiple oxidation states such as $0$, $ + 2$, $ + 4$, $ + 6$ and $ + 8$. Due to this it forms a number of oxides, some of these oxides are stable while some are not.
Xenon also forms oxide fluorides which consists of both oxygen and fluorine atoms bonded to $Xe$. One of such oxide fluorides is xenon oxytetrafluoride, i.e., $XeO{F_4}$.
The O-atom is in $ - 2$ oxidation state and each F-atom is in $ - 1$ oxidation state. The overall charge on the molecule is zero, so the oxidation state of $Xe$ is,
$x + ( - 2) + ( - 1 \times 4) = 0$
$ \Rightarrow x - 2 - 4 = 0$
$ \Rightarrow x - 6 = 0$
$ \Rightarrow x = + 6$
$Xe$ is a noble gas and it has $8$ valence electrons. As it is in $ + 6$ oxidation state, it means it has shared $6$ electrons with the bonded atoms. Therefore, only $2$ more electrons are left in a non-bonded state. That is, one lone pair of electrons is present on $Xe$.
Structure of $XeO{F_4}$:
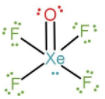
Note:
We can also find the number of lone pairs directly by calculating the number of electrons shared by $Xe$ to the bonded atoms, without the need to calculate the oxidation number. As $Xe$ has $8$ valence electrons, it is bonded to O-atom with a double bond, so $2$ electrons are shared; $4$ F-atoms are singly bonded to $Xe$, so $4$ electrons are shared with fluorine. The rest $2$ electrons are present as lone pairs of electrons.
Complete step by step answer:
Xenon is found in multiple oxidation states such as $0$, $ + 2$, $ + 4$, $ + 6$ and $ + 8$. Due to this it forms a number of oxides, some of these oxides are stable while some are not.
Xenon also forms oxide fluorides which consists of both oxygen and fluorine atoms bonded to $Xe$. One of such oxide fluorides is xenon oxytetrafluoride, i.e., $XeO{F_4}$.
The O-atom is in $ - 2$ oxidation state and each F-atom is in $ - 1$ oxidation state. The overall charge on the molecule is zero, so the oxidation state of $Xe$ is,
$x + ( - 2) + ( - 1 \times 4) = 0$
$ \Rightarrow x - 2 - 4 = 0$
$ \Rightarrow x - 6 = 0$
$ \Rightarrow x = + 6$
$Xe$ is a noble gas and it has $8$ valence electrons. As it is in $ + 6$ oxidation state, it means it has shared $6$ electrons with the bonded atoms. Therefore, only $2$ more electrons are left in a non-bonded state. That is, one lone pair of electrons is present on $Xe$.
Structure of $XeO{F_4}$:

Note:
We can also find the number of lone pairs directly by calculating the number of electrons shared by $Xe$ to the bonded atoms, without the need to calculate the oxidation number. As $Xe$ has $8$ valence electrons, it is bonded to O-atom with a double bond, so $2$ electrons are shared; $4$ F-atoms are singly bonded to $Xe$, so $4$ electrons are shared with fluorine. The rest $2$ electrons are present as lone pairs of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




