
Find the number of functional groups in the given compound.
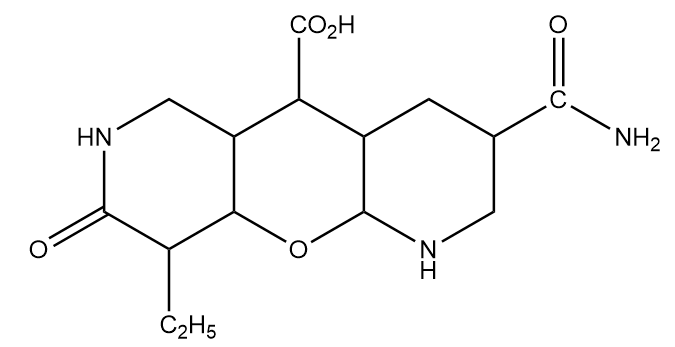

Answer
510.3k+ views
Hint: In organic chemistry, a functional group is a specific group of atoms or molecules which are bonded to compounds at a particular position. These groups account for different characteristic physical and chemical properties of compounds and are responsible to undergo distinctive chemical reactions.
Complete answer:
In the structure of the given compound, the functional groups are represented within the red circle as follows:
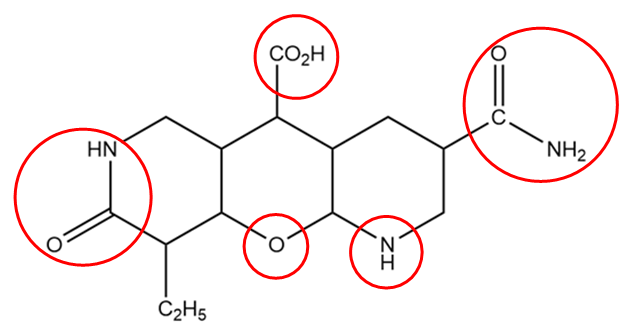
The general representation and name of the functional groups in the given compound are as follows:
1. Primary amide: The general representation of this group is as follows:
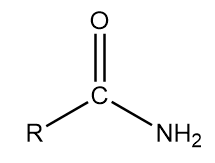
Where, R is an alkyl group and $ CON{H_2} $ part of the structure contributes to the functional group of the compound.
2. Secondary amine: The general representation of this group is $ {R_1} - NH - {R_2} $ , where $ {R_1} $ and $ {R_2} $ are the alkyl groups which can either be same or different and $ - NH - $ part of the structure contributes to the functional group of the compound.
3. Ether: The general representation of this group is $ {R_1} - O - {R_2} $ , where $ {R_1} $ and $ {R_2} $ are the alkyl groups which can either be same or different and $ - O - $ part of the structure contributes to the functional group of the compound.
4. Secondary amide: The general representation of this group is as follows:
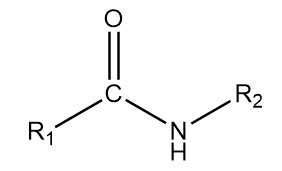
where $ {R_1} $ and $ {R_2} $ are the alkyl groups which can either be same or different and the $ - CONH - $ part of the structure contributes to the functional group of the compound.
5. Carboxylic acid: The general representation of this group is as follows:
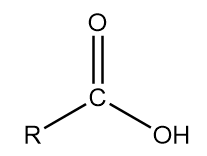
Where, R is an alkyl group and $ COOH $ part of the structure contributes to the functional group of the compound.
Hence, the total number of functional groups or substituent groups present in the given compound is $ = 5 $ .
Note:
Always remember that the alkanes are not considered as a part of functional groups in an organic compound because alkanes consist of carbon-carbon single bonds which have only one sigma bond and are much stronger and stable than alkene and alkyne molecules. Therefore, in the given compound ethyl group i.e., $ {C_2}{H_5} $ group is not considered as a functional group.
Complete answer:
In the structure of the given compound, the functional groups are represented within the red circle as follows:

The general representation and name of the functional groups in the given compound are as follows:
1. Primary amide: The general representation of this group is as follows:

Where, R is an alkyl group and $ CON{H_2} $ part of the structure contributes to the functional group of the compound.
2. Secondary amine: The general representation of this group is $ {R_1} - NH - {R_2} $ , where $ {R_1} $ and $ {R_2} $ are the alkyl groups which can either be same or different and $ - NH - $ part of the structure contributes to the functional group of the compound.
3. Ether: The general representation of this group is $ {R_1} - O - {R_2} $ , where $ {R_1} $ and $ {R_2} $ are the alkyl groups which can either be same or different and $ - O - $ part of the structure contributes to the functional group of the compound.
4. Secondary amide: The general representation of this group is as follows:

where $ {R_1} $ and $ {R_2} $ are the alkyl groups which can either be same or different and the $ - CONH - $ part of the structure contributes to the functional group of the compound.
5. Carboxylic acid: The general representation of this group is as follows:

Where, R is an alkyl group and $ COOH $ part of the structure contributes to the functional group of the compound.
Hence, the total number of functional groups or substituent groups present in the given compound is $ = 5 $ .
Note:
Always remember that the alkanes are not considered as a part of functional groups in an organic compound because alkanes consist of carbon-carbon single bonds which have only one sigma bond and are much stronger and stable than alkene and alkyne molecules. Therefore, in the given compound ethyl group i.e., $ {C_2}{H_5} $ group is not considered as a functional group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




