
Find the number of delocalized electrons in naphthalene molecules.
Answer
589.5k+ views
Hint: First of all, we need to draw the structure of naphthalene. The structure of naphthalene consists of two fused benzene rings. Delocalized electrons are those electrons which take part in resonance, and are distributed throughout the entire molecule.
Complete step by step answer:
Structure of naphthalene is given below,
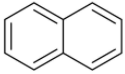
The resonance in naphthalene molecules involves the delocalization of all the $\pi - $electrons present. As only those bonds take part in resonance which are present in conjugation with each other and in this molecule all the pi-bonds are in conjugation with each other.
The resonating structures or canonical forms of naphthalene are shown below.
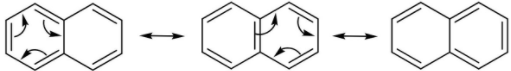
The number of pi-bonds taking part in resonance are $5$.
As we know the number of $\pi - $electrons participating in resonance are double the number of pi-bonds. So,
Number of electrons participating in resonance, that is, number of electrons that are delocalized in the naphthalene molecule are
$ = 5 \times 2$
$ \Rightarrow 10$
Note:
The resonating structures show the delocalization of electrons within the molecule. In these structures the number of atoms within the molecule and the total number of electrons remain the same. The overall charge on the molecule also remains the same, while the charge separation and distribution may change.
Complete step by step answer:
Structure of naphthalene is given below,

The resonance in naphthalene molecules involves the delocalization of all the $\pi - $electrons present. As only those bonds take part in resonance which are present in conjugation with each other and in this molecule all the pi-bonds are in conjugation with each other.
The resonating structures or canonical forms of naphthalene are shown below.

The number of pi-bonds taking part in resonance are $5$.
As we know the number of $\pi - $electrons participating in resonance are double the number of pi-bonds. So,
Number of electrons participating in resonance, that is, number of electrons that are delocalized in the naphthalene molecule are
$ = 5 \times 2$
$ \Rightarrow 10$
Note:
The resonating structures show the delocalization of electrons within the molecule. In these structures the number of atoms within the molecule and the total number of electrons remain the same. The overall charge on the molecule also remains the same, while the charge separation and distribution may change.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




