
Find the number of delocalized electrons in naphthalene molecules.
Answer
579.9k+ views
Hint: First of all, we need to draw the structure of naphthalene. The structure of naphthalene consists of two fused benzene rings. Delocalized electrons are those electrons which take part in resonance, and are distributed throughout the entire molecule.
Complete step by step answer:
Structure of naphthalene is given below,
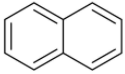
The resonance in naphthalene molecules involves the delocalization of all the $\pi - $electrons present. As only those bonds take part in resonance which are present in conjugation with each other and in this molecule all the pi-bonds are in conjugation with each other.
The resonating structures or canonical forms of naphthalene are shown below.
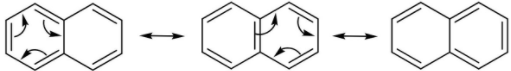
The number of pi-bonds taking part in resonance are $5$.
As we know the number of $\pi - $electrons participating in resonance are double the number of pi-bonds. So,
Number of electrons participating in resonance, that is, number of electrons that are delocalized in the naphthalene molecule are
$ = 5 \times 2$
$ \Rightarrow 10$
Note:
The resonating structures show the delocalization of electrons within the molecule. In these structures the number of atoms within the molecule and the total number of electrons remain the same. The overall charge on the molecule also remains the same, while the charge separation and distribution may change.
Complete step by step answer:
Structure of naphthalene is given below,

The resonance in naphthalene molecules involves the delocalization of all the $\pi - $electrons present. As only those bonds take part in resonance which are present in conjugation with each other and in this molecule all the pi-bonds are in conjugation with each other.
The resonating structures or canonical forms of naphthalene are shown below.

The number of pi-bonds taking part in resonance are $5$.
As we know the number of $\pi - $electrons participating in resonance are double the number of pi-bonds. So,
Number of electrons participating in resonance, that is, number of electrons that are delocalized in the naphthalene molecule are
$ = 5 \times 2$
$ \Rightarrow 10$
Note:
The resonating structures show the delocalization of electrons within the molecule. In these structures the number of atoms within the molecule and the total number of electrons remain the same. The overall charge on the molecule also remains the same, while the charge separation and distribution may change.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




