
Find the n factor of nitrobenzene.
A.$3$
B.$6$
C.$2$
D.$4$

Answer
522.9k+ views
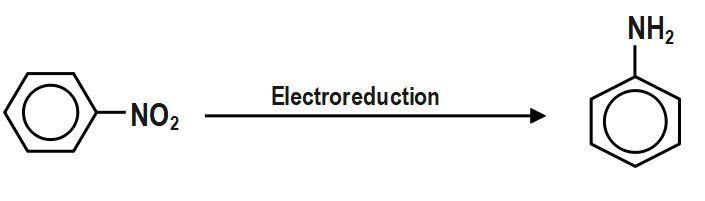
Hint: We must know that nitrobenzene to aniline conversion by reduction is possible by electrolytic reduction method in a weakly acidic medium. In this question, we have to out the value of n, i.e. valency factor. We should be taken to avoid further reduction to cyclohexylamine.
Complete answer:
The nitro benzene is prepared from benzene by the process of nitration. Nitration is the process, where a nitro group substitutes the hydrogen of the aromatic ring compound by reacting with nitric acid and sulphuric acid. First in the reaction, nitric acid reacts with sulphuric acid to form nitronium ion.
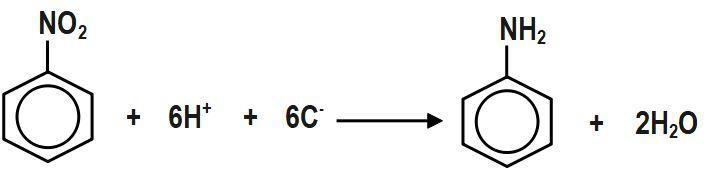
As we know, Nitrobenzene can be reduced to aniline in the presence of metal or acid by electrolytic reduction method in a weakly acidic medium. So, as given, the electrolytic reduction reaction for nitrobenzene, the reduction of one molecule of nitrobenzene to aniline requires 6 hydrogen atoms, so for this reaction, the valency factor or n is equal to \[6.\]
In the electrolytic reduction of nitrobenzene to aniline reaction, \[6{{e}^{-}}\] is consumed by nitrobenzene to get reduced into aniline. Thus n factor of nitrobenzene in the above reaction is \[6.\]
Therefore, the correct answer is Option B.
Note:
We must know that aniline is an important industrial chemical product. This is used on a large scale to produce polyurethane. We can produce aniline by hydrogenation of nitrobenzene with raney nickel or nickel alloy in large scale. If we prepare aniline by electrochemical reduction of nitrobenzene.
Complete answer:
The nitro benzene is prepared from benzene by the process of nitration. Nitration is the process, where a nitro group substitutes the hydrogen of the aromatic ring compound by reacting with nitric acid and sulphuric acid. First in the reaction, nitric acid reacts with sulphuric acid to form nitronium ion.
As we know, Nitrobenzene can be reduced to aniline in the presence of metal or acid by electrolytic reduction method in a weakly acidic medium. So, as given, the electrolytic reduction reaction for nitrobenzene, the reduction of one molecule of nitrobenzene to aniline requires 6 hydrogen atoms, so for this reaction, the valency factor or n is equal to \[6.\]
In the electrolytic reduction of nitrobenzene to aniline reaction, \[6{{e}^{-}}\] is consumed by nitrobenzene to get reduced into aniline. Thus n factor of nitrobenzene in the above reaction is \[6.\]

Therefore, the correct answer is Option B.
Note:
We must know that aniline is an important industrial chemical product. This is used on a large scale to produce polyurethane. We can produce aniline by hydrogenation of nitrobenzene with raney nickel or nickel alloy in large scale. If we prepare aniline by electrochemical reduction of nitrobenzene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




