
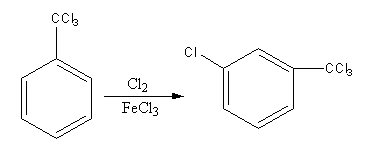
Find the major product in the following reaction:
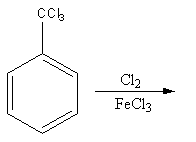
A)
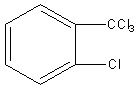
B)
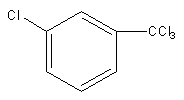
C)
D)
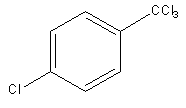





Answer
578.1k+ views
Hint: The reagent ${\text{C}}{{\text{l}}_{\text{2}}}{\text{/FeC}}{{\text{l}}_{\text{3}}}$ is used for the chlorination. We can decide the site of chlorination by checking the directing effect of ${\text{CC}}{{\text{l}}_3}$ group. The ${\text{CC}}{{\text{l}}_3}$ group is a meta-directing group.
Complete answer:
The chlorine in presence of ferric chloride is used for chlorination. The addition of chlorine is known as chlorination. The chlorination is an electrophilic substitution reaction.
The electrophile chlorine attacks on the benzene ring and then hydrogen removes to give a product.
The chlorination can occur at any position in the benzene ring. But if any group is present on the benzene ring the group causes the partial charge separation, so it directs the attacking group according to its nature on the benzene ring.
${\text{CC}}{{\text{l}}_3}$ group has $ - {\text{R}}$ effect so, it attracts the electrons towards itself thus, increase the electron density on meta position and decrease the electron density on ortho and para position.
So, electrophile chlorine attacks at the meta position, so the product of the reaction will be meta-chlorinated.
So, the product of the reaction is,
Therefore, option (B) is correct.
Note: The groups that withdraw the electron density form the ring show the $ - {\text{R}}$ effect whereas the groups that donate the electron density to the ring show the ${\text{ + R}}$ effect. The groups that show$ - {\text{R}}$ effect work as meta directing for an electrophile and the groups that show ${\text{ + R}}$ effect work as ortho and para directing for an electrophile.
Complete answer:
The chlorine in presence of ferric chloride is used for chlorination. The addition of chlorine is known as chlorination. The chlorination is an electrophilic substitution reaction.
The electrophile chlorine attacks on the benzene ring and then hydrogen removes to give a product.
The chlorination can occur at any position in the benzene ring. But if any group is present on the benzene ring the group causes the partial charge separation, so it directs the attacking group according to its nature on the benzene ring.
${\text{CC}}{{\text{l}}_3}$ group has $ - {\text{R}}$ effect so, it attracts the electrons towards itself thus, increase the electron density on meta position and decrease the electron density on ortho and para position.
So, electrophile chlorine attacks at the meta position, so the product of the reaction will be meta-chlorinated.
So, the product of the reaction is,

Therefore, option (B) is correct.
Note: The groups that withdraw the electron density form the ring show the $ - {\text{R}}$ effect whereas the groups that donate the electron density to the ring show the ${\text{ + R}}$ effect. The groups that show$ - {\text{R}}$ effect work as meta directing for an electrophile and the groups that show ${\text{ + R}}$ effect work as ortho and para directing for an electrophile.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




