
Find out the major product of the following reaction:
\[C{H_3} - C{H_2} - CO - C{H_3}\] \[\xrightarrow{{[O]}}\]
Answer
508.2k+ views
Hint :We are given a ketone and we have to find the product when it is oxidised. We know that when ketones are oxidised they produce carboxylic acids. Ketones are fairly resistant to oxidation because ketones do not have hydrogen atoms attached to their carbonyl carbon. The oxidation of ketone occurs under drastic conditions and requires strong oxidising agents.
Some of the important strong oxidising agents are acidified $ {K_2}C{r_2}{O_7} $ and alkaline $ KMn{O_4} $ solution.
Complete Step By Step Answer:
When unsymmetrical ketones are subjected to oxidation they will produce a mixture of carboxylic acids.
By looking at the cleavage of ketones we will be able to identify which all acids are obtained.
In general if we take a ketone
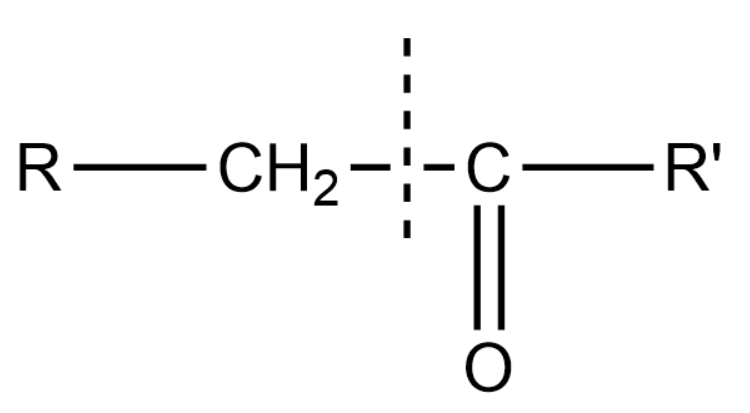
Here $ R' $ and $ R' $ are two different alkyl groups.
When we treat these kind of ketones to oxidising agents we will see that they break into two different carboxylic acids which can be denoted by $ R - COOH $ and $ R' - COOH $
One part is getting an $ - OH $ group whereas the other $ - C{H_2} $ group is getting oxidised to the $ - COOH $ group.
So in this question since we are taking a butanone we will get ethanoic acid as the product of oxidation.
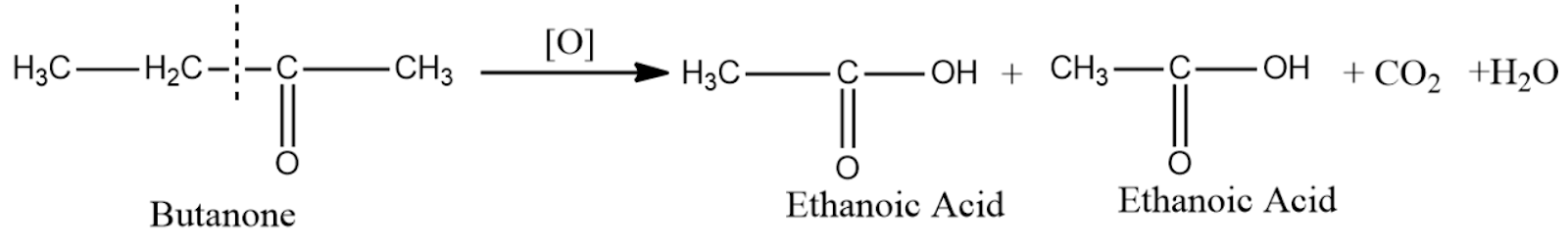
Note :
The oxidation of Ketones results in the formation of carboxylic acids with a lesser number of Carbon atoms.
Oxidation of symmetric ketones will always yield only one type of carboxylic acid, $ C{O_2} $ and $ {H_2}O $ . In that case, the cleavage occurs at the carbonyl carbon and one of the hydrocarbon chain parts will be oxidized to give $ C{O_2} $ and $ {H_2}O $ .
Oxidation of cyclohexane in presence of concentrated nitric acid results in the formation of adipic acid.
Some of the important strong oxidising agents are acidified $ {K_2}C{r_2}{O_7} $ and alkaline $ KMn{O_4} $ solution.
Complete Step By Step Answer:
When unsymmetrical ketones are subjected to oxidation they will produce a mixture of carboxylic acids.
By looking at the cleavage of ketones we will be able to identify which all acids are obtained.
In general if we take a ketone

Here $ R' $ and $ R' $ are two different alkyl groups.
When we treat these kind of ketones to oxidising agents we will see that they break into two different carboxylic acids which can be denoted by $ R - COOH $ and $ R' - COOH $
One part is getting an $ - OH $ group whereas the other $ - C{H_2} $ group is getting oxidised to the $ - COOH $ group.
So in this question since we are taking a butanone we will get ethanoic acid as the product of oxidation.

Note :
The oxidation of Ketones results in the formation of carboxylic acids with a lesser number of Carbon atoms.
Oxidation of symmetric ketones will always yield only one type of carboxylic acid, $ C{O_2} $ and $ {H_2}O $ . In that case, the cleavage occurs at the carbonyl carbon and one of the hydrocarbon chain parts will be oxidized to give $ C{O_2} $ and $ {H_2}O $ .
Oxidation of cyclohexane in presence of concentrated nitric acid results in the formation of adipic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




