
What is the final product obtained in the reaction between ethane nitrile and ethyl magnesium bromide?
A.Methoxy propane
B.Butanamine
C.Butan-2-one
D.Pent-2-one
Answer
597.9k+ views
Hint: The chemical compounds which have the general formula ${\text{R - Mg - X}}$ where X represents a halogen and R represents an organic group, which may be alkyl or aryl, are termed as ‘Grignard reagents’.
Aliphatic ketones can be prepared by the action of a suitable Grignard reagent on an alkyl nitrile. This method of preparation is useful for preparing ketones only.
Complete step by step answer:
We need to determine what the final product is in the reaction between ethane nitrile and ethyl magnesium bromide.
Ethane nitrile, also known as methyl cyanide or acetonitrile is the simplest organic nitrile and has the molecular formula ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CN}}$ .
Ethyl magnesium bromide is a Grignard reagent having the formula ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}}$ .
Thus, when ethane nitrile is acted upon by ethyl magnesium bromide, the strongly organometallic reagent adds to the carbon-nitrogen triple bond in a way similar to aldehydes and ketones. The reaction first proceeds through an imine salt intermediate which is then hydrolysed to give butan-2-one.
butan-2-one.
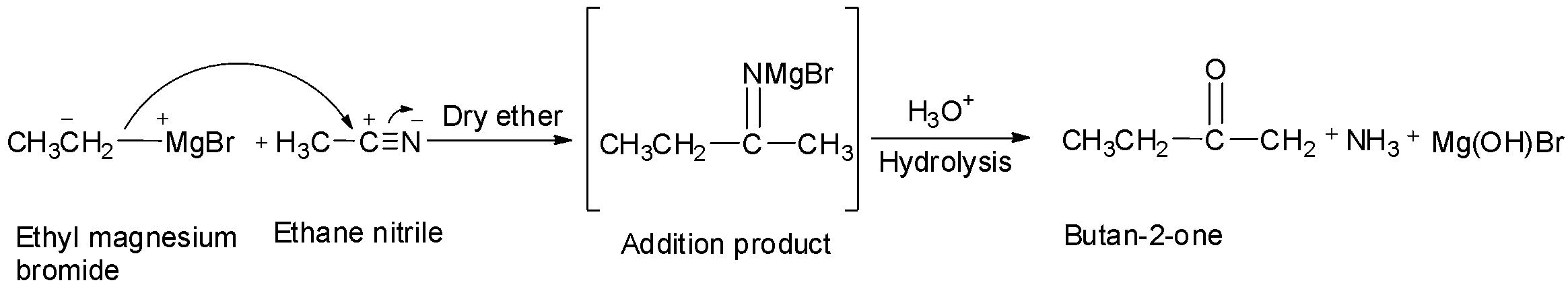
Therefore, option C is correct.
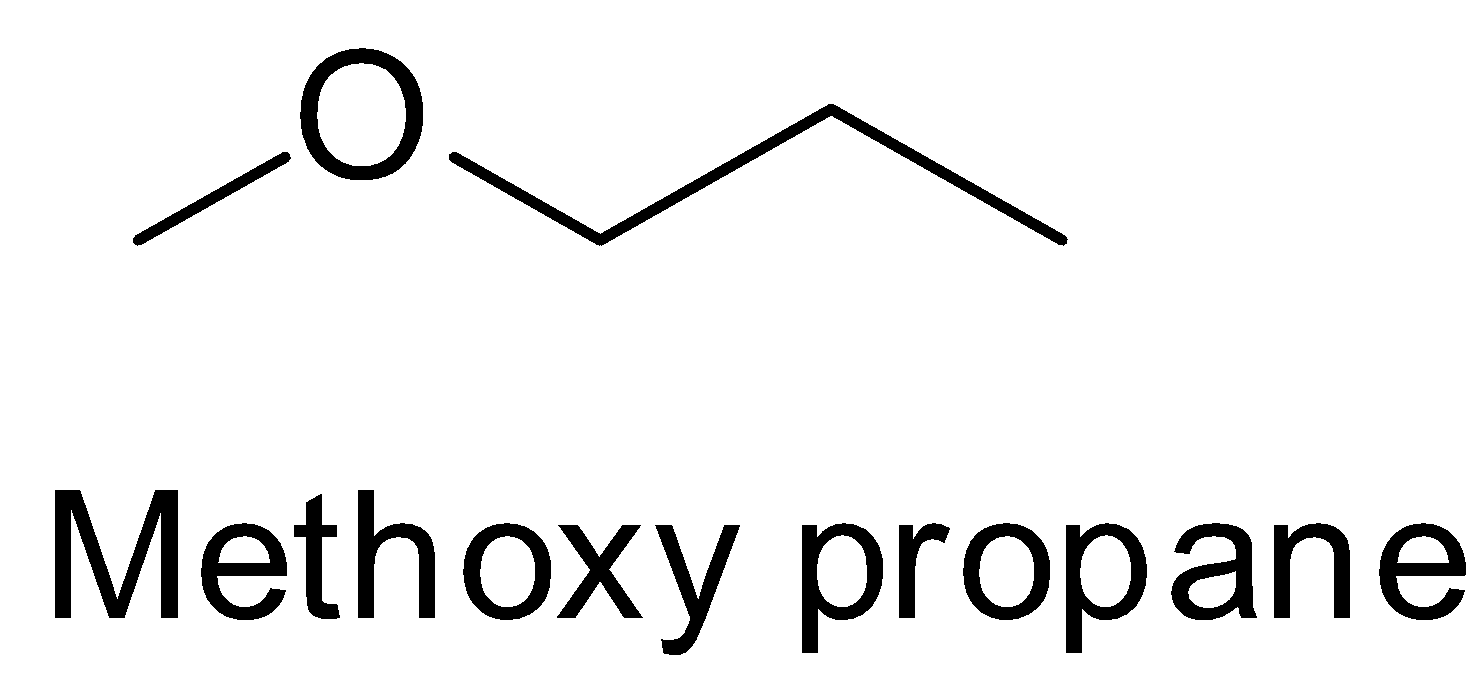
Methoxy propane or methyl propyl ether is an ether, not a ketone. So it cannot be the product of the reaction between a nitrile and a Grignard reagent. So, A is incorrect.
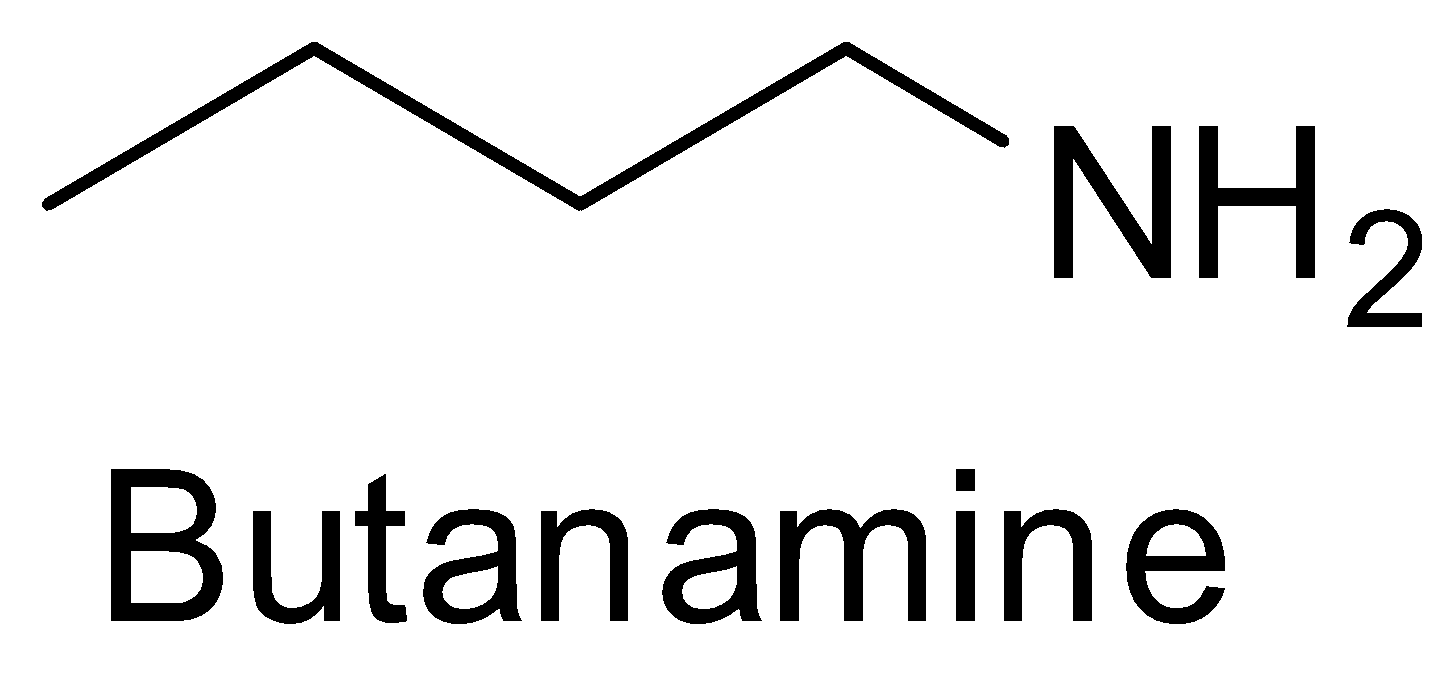
Butanamine is an amine of butane and so it cannot be the product of the reaction between a nitrile and a Grignard reagent. So, B is incorrect.
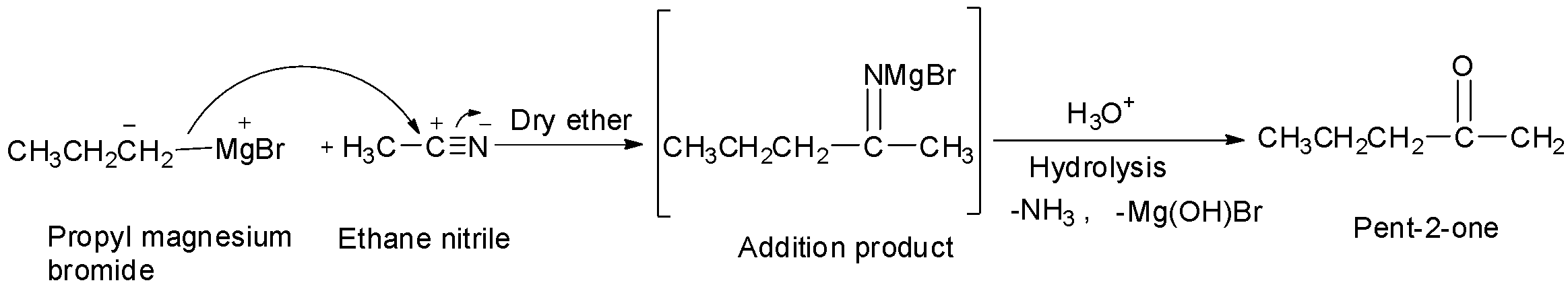
Pent-2-one is methyl propyl ketone and it will be formed when propyl magnesium bromide reacts with ethane nitrile. So. D is also incorrect.
Hence option C is correct.
Note:
If hydrocyanic acid is used instead of nitrile, then aldehyde is formed.
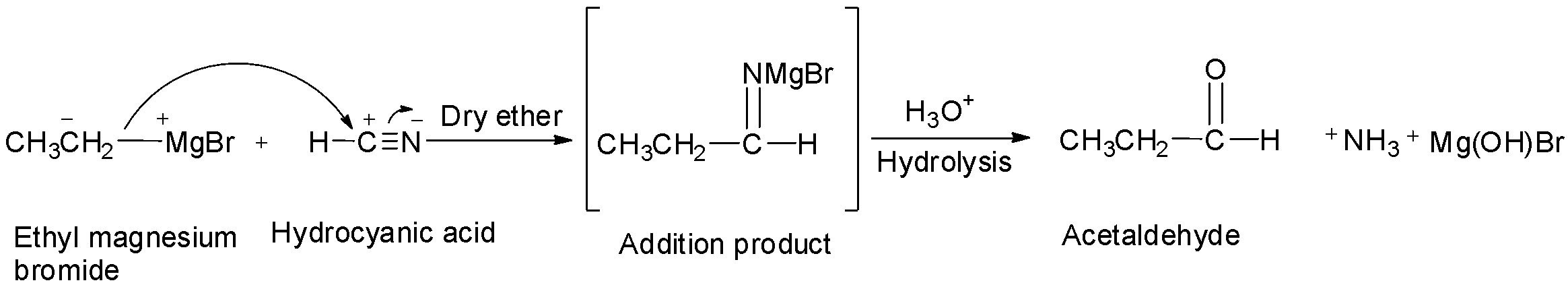
Aliphatic ketones can be prepared by the action of a suitable Grignard reagent on an alkyl nitrile. This method of preparation is useful for preparing ketones only.
Complete step by step answer:
We need to determine what the final product is in the reaction between ethane nitrile and ethyl magnesium bromide.
Ethane nitrile, also known as methyl cyanide or acetonitrile is the simplest organic nitrile and has the molecular formula ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CN}}$ .
Ethyl magnesium bromide is a Grignard reagent having the formula ${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{MgBr}}$ .
Thus, when ethane nitrile is acted upon by ethyl magnesium bromide, the strongly organometallic reagent adds to the carbon-nitrogen triple bond in a way similar to aldehydes and ketones. The reaction first proceeds through an imine salt intermediate which is then hydrolysed to give butan-2-one.
butan-2-one.

Therefore, option C is correct.
Methoxy propane or methyl propyl ether is an ether, not a ketone. So it cannot be the product of the reaction between a nitrile and a Grignard reagent. So, A is incorrect.

Butanamine is an amine of butane and so it cannot be the product of the reaction between a nitrile and a Grignard reagent. So, B is incorrect.

Pent-2-one is methyl propyl ketone and it will be formed when propyl magnesium bromide reacts with ethane nitrile. So. D is also incorrect.

Hence option C is correct.
Note:
If hydrocyanic acid is used instead of nitrile, then aldehyde is formed.

Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




