
What is the final product in the following reaction:
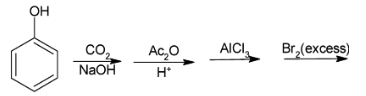

Answer
552.3k+ views
Hint:In the above question, it is asked about the final product formed when phenol undergoes through the above reagent. The first 3 steps come under Kolbe’s reaction and addition of excess bromine adds bromine to ortho and para positions.
Complete step-by-step answer:Since, in the above question, the reagents used are mainly the reagents used in Kolbe’s reaction, so let us discuss about Kolbe’s reaction. Kolbe’s reaction is the reaction in which carboxylic acid is added onto the benzene ring of phenol or its derivative.
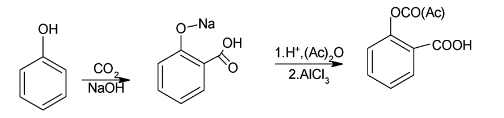
When phenol is added to carbon-dioxide and sodium hydroxide, it leads addition of carbon dioxide on the benzene ring which is negatively charged which results in formation of sodium salicylate.
When sodium salicylate undergoes acidification and acetylation, it leads to the formation of salicylic acid. Salicylic acid when reacts with lewis acid like ${\text{FeC}}{{\text{l}}_{\text{3}}}$, ${\text{AlC}}{{\text{l}}_{\text{3}}}$ etc. forms acetyl salicylic acid.
Since acetyl salicylic acid reacts with excess bromine and gets hydrolysed leads to the formation of salicylic acid which when reacts with bromine to form 2,4,6 tribromophenol.
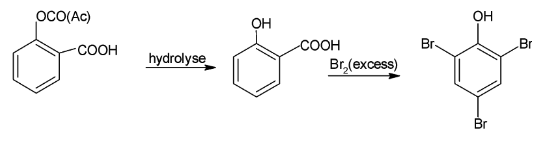
Hence, the final product formed is 2,4,6 tribromophenol.
Note:Aspirin, which is also known as salicylate, is used to reduce fever and relieve mild to moderate pain from conditions such as muscle aches, toothaches, common cold and headaches. It may also be used to reduce pain and swelling in conditions.
Aspirin can help in preventing heart attack or clot-related stroke by interfering with how the blood clots. But this same property makes aspirin work as a blood thinner to stop it from clotting as it may also cause unwanted side effects, which includes bleeding into the brain or stomach.
Complete step-by-step answer:Since, in the above question, the reagents used are mainly the reagents used in Kolbe’s reaction, so let us discuss about Kolbe’s reaction. Kolbe’s reaction is the reaction in which carboxylic acid is added onto the benzene ring of phenol or its derivative.
When phenol is added to carbon-dioxide and sodium hydroxide, it leads addition of carbon dioxide on the benzene ring which is negatively charged which results in formation of sodium salicylate.
When sodium salicylate undergoes acidification and acetylation, it leads to the formation of salicylic acid. Salicylic acid when reacts with lewis acid like ${\text{FeC}}{{\text{l}}_{\text{3}}}$, ${\text{AlC}}{{\text{l}}_{\text{3}}}$ etc. forms acetyl salicylic acid.

Since acetyl salicylic acid reacts with excess bromine and gets hydrolysed leads to the formation of salicylic acid which when reacts with bromine to form 2,4,6 tribromophenol.

Hence, the final product formed is 2,4,6 tribromophenol.
Note:Aspirin, which is also known as salicylate, is used to reduce fever and relieve mild to moderate pain from conditions such as muscle aches, toothaches, common cold and headaches. It may also be used to reduce pain and swelling in conditions.
Aspirin can help in preventing heart attack or clot-related stroke by interfering with how the blood clots. But this same property makes aspirin work as a blood thinner to stop it from clotting as it may also cause unwanted side effects, which includes bleeding into the brain or stomach.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




