
Fill in the blanks: The oxidation state of nitrogen in ammonia molecules is ________.
Answer
585.9k+ views
Hint: The oxidation state, sometimes referred to as oxidation number, describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
Complete step by step answer:
We have been provided with ammonia whose formula is $N{H_3} $ and structure,
We need to calculate the oxidation number of nitrogen in ammonia molecule:
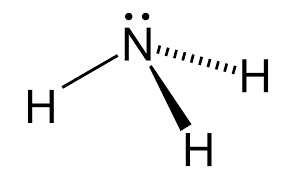
Oxidation number, also called oxidation state, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
Now, let the oxidation state of nitrogen in ammonia that is $N{H_3} $ be x.
We know that the oxidation state of hydrogen is +1.
So, the equation would be: $x + 3(+ 1) = 0$,
Simplifying the equation: $x + 3 = 0$,
The value of x comes out to be: $x = - 3$.
So, therefore we can say that the oxidation state of nitrogen in ammonia is -3.
Note: An oxidation state shows how many electrons an atom would gain or lose if it were to bond with other atoms. Transition metals can have multiple oxidation states because of their electrons. When they attach to other atoms, some of their electrons change energy levels. Atoms of a higher positive oxidation state exhibit a higher binding energy due to the extra coulombic interaction between the photon emitted electron and the ion core
Complete step by step answer:
We have been provided with ammonia whose formula is $N{H_3} $ and structure,
We need to calculate the oxidation number of nitrogen in ammonia molecule:

Oxidation number, also called oxidation state, the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom.
Now, let the oxidation state of nitrogen in ammonia that is $N{H_3} $ be x.
We know that the oxidation state of hydrogen is +1.
So, the equation would be: $x + 3(+ 1) = 0$,
Simplifying the equation: $x + 3 = 0$,
The value of x comes out to be: $x = - 3$.
So, therefore we can say that the oxidation state of nitrogen in ammonia is -3.
Note: An oxidation state shows how many electrons an atom would gain or lose if it were to bond with other atoms. Transition metals can have multiple oxidation states because of their electrons. When they attach to other atoms, some of their electrons change energy levels. Atoms of a higher positive oxidation state exhibit a higher binding energy due to the extra coulombic interaction between the photon emitted electron and the ion core
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




