
Explain why the angle of contact is acute for the kerosene-glass pair and is obtuse for mercury-glass pairs.
Answer
567.9k+ views
Hint:The angle of contact or contact angle is the angle that is measured through the liquid from the liquid-solid interface. It is used to measure the wettability of the solid surface by the liquid using the young’s equation.
Complete answer:
Consider kerosene which is in contact with the glass.
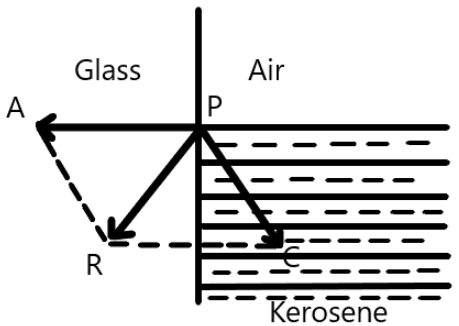
Here, $P$ is the molecule that is deflected in the liquid.
Here, the adhesive force between the pair will be very large as compared to the cohesive force. Therefore, the resultant of the forces will be directed into the solid. Now, as we know, the free surface of a liquid that is at rest will be perpendicular to the resultant of the force on it. Therefore, the molecule $P$ will move upward until the surface of the liquid will become perpendicular to the $P\vec R$ . Therefore, the shape of the liquid will become concave and the tangent to the surface will become an acute angle with the surface.
Now, consider mercury which is in contact with the glass pair.

Now, as we know that mercury is a non-wetting liquid, therefore, the cohesive force between the pair will be large as compared to the adhesive force. Therefore, the resultant of the forces will be towards liquid. The molecule $P$ will move downwards until the surface of the liquid will become perpendicular to $P\vec R$ . Hence, the liquid will acquire a convex shape and the tangent to the surface will become an obtuse angle with the surface of the liquid.
Hence, the angle of the kerosene-glass pair is an acute angle and the angle of the mercury-glass pair is an obtuse angle.
Note:Now, you might get confused about acute and obtuse angles. Just remember, surface tension is inversely proportional to the angle of contact. Therefore, with the decrease in surface tension, the temperature will increase which results in a decrease in the intermolecular cohesive force. Therefore, the angle of contact will decrease making an acute angle. Now, with the increase in surface tension, the temperature will decrease which results in a decrease in the intermolecular adhesive force. Therefore, the angle of contact will increase making it obtuse.
Complete answer:
Consider kerosene which is in contact with the glass.

Here, $P$ is the molecule that is deflected in the liquid.
Here, the adhesive force between the pair will be very large as compared to the cohesive force. Therefore, the resultant of the forces will be directed into the solid. Now, as we know, the free surface of a liquid that is at rest will be perpendicular to the resultant of the force on it. Therefore, the molecule $P$ will move upward until the surface of the liquid will become perpendicular to the $P\vec R$ . Therefore, the shape of the liquid will become concave and the tangent to the surface will become an acute angle with the surface.
Now, consider mercury which is in contact with the glass pair.

Now, as we know that mercury is a non-wetting liquid, therefore, the cohesive force between the pair will be large as compared to the adhesive force. Therefore, the resultant of the forces will be towards liquid. The molecule $P$ will move downwards until the surface of the liquid will become perpendicular to $P\vec R$ . Hence, the liquid will acquire a convex shape and the tangent to the surface will become an obtuse angle with the surface of the liquid.
Hence, the angle of the kerosene-glass pair is an acute angle and the angle of the mercury-glass pair is an obtuse angle.
Note:Now, you might get confused about acute and obtuse angles. Just remember, surface tension is inversely proportional to the angle of contact. Therefore, with the decrease in surface tension, the temperature will increase which results in a decrease in the intermolecular cohesive force. Therefore, the angle of contact will decrease making an acute angle. Now, with the increase in surface tension, the temperature will decrease which results in a decrease in the intermolecular adhesive force. Therefore, the angle of contact will increase making it obtuse.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




