
Explain why ortho-nitrophenol is more acidic than ortho-methoxyphenol?
Answer
591.6k+ views
Hint: A compound is said to be acidic based on its ability to donate a proton or hydrogen atom. The more acidic compound will easily donate the proton and form a stable ionic form which is stabilized by factors such as resonance.
Complete step by step answer:
1) First of all, we will discuss the concept of acidity which will simplify the approach to solve the question. The acidity of a compound is its ability to donate the proton or hydrogen in a reaction.
2) The compounds which donate protons more easily will be considered as the most acidic compounds.
3) Let's see the factor which makes the molecule donate the proton easily, which is the formation of a stable conjugate base. When a proton is donated it will form the anionic form of the molecule which is called the conjugate base of that acid.
4) A conjugate base is said to be stable based on its ability to stabilize the anion present on that molecule. Factors such as resonance in a molecule and the presence of an electron-withdrawing group in a molecule will make an anion stabilize in the structure.
5) Now let's see the structures of ortho-nitrophenol and ortho-methoxyphenol,
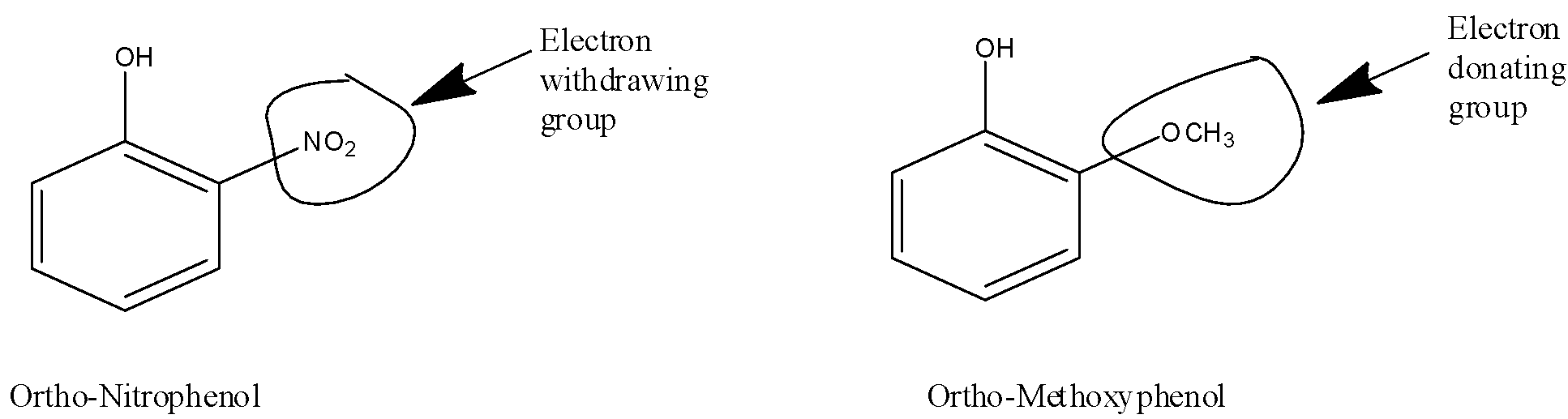
An electron-withdrawing group is a group that has the ability to pull the energy of the electron towards itself through bonds. The structure ortho-nitro phenol has an electron-withdrawing group $ - N{O_2}$ that stabilizes the negative ion on oxygen after donating the hydrogen atom. Also, the negative ion on the oxygen gets stabilized due to the resonance effect in the structure. The stabilized anion on the structure will make it more acidic.
6) While in ortho-methoxyphenol the $ - OC{H_3}$ group is an electron-donating group that donates the electron energy to the structure further making the anion on oxygen more unstabilized. The unstabilized anion on the structure will make the structure less acidic.
7) Therefore, by analyzing the above data we have come to the conclusion that the ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Note:
The acidity of a compound is dependent on the stability of its conjugate base. The presence of an electron-withdrawing group and resonance in the structure will also increase the acidity of the compound. For a structure to have resonance there must be the presence of conjugation i.e. alternate double bonds.
Complete step by step answer:
1) First of all, we will discuss the concept of acidity which will simplify the approach to solve the question. The acidity of a compound is its ability to donate the proton or hydrogen in a reaction.
2) The compounds which donate protons more easily will be considered as the most acidic compounds.
3) Let's see the factor which makes the molecule donate the proton easily, which is the formation of a stable conjugate base. When a proton is donated it will form the anionic form of the molecule which is called the conjugate base of that acid.
4) A conjugate base is said to be stable based on its ability to stabilize the anion present on that molecule. Factors such as resonance in a molecule and the presence of an electron-withdrawing group in a molecule will make an anion stabilize in the structure.
5) Now let's see the structures of ortho-nitrophenol and ortho-methoxyphenol,

An electron-withdrawing group is a group that has the ability to pull the energy of the electron towards itself through bonds. The structure ortho-nitro phenol has an electron-withdrawing group $ - N{O_2}$ that stabilizes the negative ion on oxygen after donating the hydrogen atom. Also, the negative ion on the oxygen gets stabilized due to the resonance effect in the structure. The stabilized anion on the structure will make it more acidic.
6) While in ortho-methoxyphenol the $ - OC{H_3}$ group is an electron-donating group that donates the electron energy to the structure further making the anion on oxygen more unstabilized. The unstabilized anion on the structure will make the structure less acidic.
7) Therefore, by analyzing the above data we have come to the conclusion that the ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Note:
The acidity of a compound is dependent on the stability of its conjugate base. The presence of an electron-withdrawing group and resonance in the structure will also increase the acidity of the compound. For a structure to have resonance there must be the presence of conjugation i.e. alternate double bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




