
Explain why
(a) The angle of contact of mercury with glass is obtuse, while that of water with glass is acute.
(b) Water on a clean glass surface tends to spread out while mercury on the same surface tends to form drops. (Put differently, water wets glass while mercury does not).
(c) Surface tension of a liquid is independent of the area of the surface.
(d) Water with detergent dissolved in it should have small angles of contact.
(e) A drop of liquid under no external forces is always spherical in shape.
Answer
597k+ views
Hint: All of the statements given above are related to the surface tension of a liquid. The surface is the force per unit length felt by the molecules at the open surface. Due to surface tension, a liquid tends to occupy a minimum surface area. The angle of contact depends on the cohesive and adhesive forces. These are some points which will help in writing the reasons.
Complete step by step solution:
(a) Let the first understand what are the cohesive forces and adhesive forces.
The forces of attraction between molecules of the same substance are called cohesive forces.
The forces of attraction between molecules of different substances are called adhesive forces.
When a liquid is poured in a cylindrical glass tube, depending on the nature of the cohesive forces between liquid molecules and adhesive forces between the glass and liquid molecules, a meniscus is formed at the top open layer of the liquid.
The angle (inside the liquid) between the tangent to the surface of the meniscus drawn at the point of contact with the glass surface and a line along the surface of the glass is called the angle of contact.
When the cohesive forces are greater than the adhesive forces, an upper meniscus is formed and hence the angle of contact is an obtuse angle.
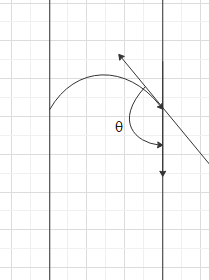
When the adhesive forces are greater than the cohesive forces, a lower meniscus is formed and hence the angle of contact is an acute angle.
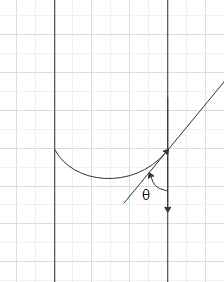
Now, we can understand that when the liquid is mercury, the cohesive forces are greater than the adhesive forces. Therefore, the angle of contact is an obtuse angle. When the liquid is water, the adhesive forces are greater than the cohesive forces. Therefore, the angle of contact is an acute angle.
(b) As discussed, the cohesive forces between the molecules of mercury are greater than the adhesive forces between the molecules of glass and mercury at the surface of contact.
The adhesive forces between the molecules of glass and water at the surface of contact are greater than the cohesive forces between the molecules of water.
Therefore, we can say that mercury likes itself more than the glass surface and thus is forms drops. Whereas water likes the surface of glass more than itself and hence it spreads on the surface of the glass.
(c) In liquids, the liquid molecules are together due to the forces of attraction between them. Therefore, the inner molecules exert a force of attraction on the molecules that are on the surface of the liquid. The force experienced by the surface molecules per unit length is called the surface tension of the liquid. Their force depends on the nature of the liquid. Thus surface tension of a liquid is independent of the area of the surface.
(d) When liquids enter (rises) through a small gap or tube, the process is called capillary rise. The water enters into the pores of the cloth due to the phenomenon of capillary rise. The capillary rise is directly proportional to the cosine of the angle of contact. We know that if the angle is small then the value of the cosine of that angle is large.
Therefore, To have a better cleaning process the water with detergent dissolved in it should have small angles of contact.
(e) Molecules of a liquid attract each other due to electromagnetic forces. Due to this, a liquid has a property of having a minimum surface area. When there are no external forces, the liquid is free to have any shape. For a given volume, the sphere is the shape with the minimum surface area. Therefore, a drop of liquid under no external forces is always spherical in shape.
Note: Students may mistake in measuring the correct angle of contact. The direction of the angle of contact from a line on the surface of the glass to the tangent at the point of contact through the liquid (as shown in the figure).
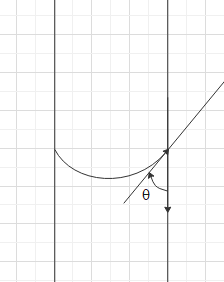
Complete step by step solution:
(a) Let the first understand what are the cohesive forces and adhesive forces.
The forces of attraction between molecules of the same substance are called cohesive forces.
The forces of attraction between molecules of different substances are called adhesive forces.
When a liquid is poured in a cylindrical glass tube, depending on the nature of the cohesive forces between liquid molecules and adhesive forces between the glass and liquid molecules, a meniscus is formed at the top open layer of the liquid.
The angle (inside the liquid) between the tangent to the surface of the meniscus drawn at the point of contact with the glass surface and a line along the surface of the glass is called the angle of contact.
When the cohesive forces are greater than the adhesive forces, an upper meniscus is formed and hence the angle of contact is an obtuse angle.

When the adhesive forces are greater than the cohesive forces, a lower meniscus is formed and hence the angle of contact is an acute angle.

Now, we can understand that when the liquid is mercury, the cohesive forces are greater than the adhesive forces. Therefore, the angle of contact is an obtuse angle. When the liquid is water, the adhesive forces are greater than the cohesive forces. Therefore, the angle of contact is an acute angle.
(b) As discussed, the cohesive forces between the molecules of mercury are greater than the adhesive forces between the molecules of glass and mercury at the surface of contact.
The adhesive forces between the molecules of glass and water at the surface of contact are greater than the cohesive forces between the molecules of water.
Therefore, we can say that mercury likes itself more than the glass surface and thus is forms drops. Whereas water likes the surface of glass more than itself and hence it spreads on the surface of the glass.
(c) In liquids, the liquid molecules are together due to the forces of attraction between them. Therefore, the inner molecules exert a force of attraction on the molecules that are on the surface of the liquid. The force experienced by the surface molecules per unit length is called the surface tension of the liquid. Their force depends on the nature of the liquid. Thus surface tension of a liquid is independent of the area of the surface.
(d) When liquids enter (rises) through a small gap or tube, the process is called capillary rise. The water enters into the pores of the cloth due to the phenomenon of capillary rise. The capillary rise is directly proportional to the cosine of the angle of contact. We know that if the angle is small then the value of the cosine of that angle is large.
Therefore, To have a better cleaning process the water with detergent dissolved in it should have small angles of contact.
(e) Molecules of a liquid attract each other due to electromagnetic forces. Due to this, a liquid has a property of having a minimum surface area. When there are no external forces, the liquid is free to have any shape. For a given volume, the sphere is the shape with the minimum surface area. Therefore, a drop of liquid under no external forces is always spherical in shape.
Note: Students may mistake in measuring the correct angle of contact. The direction of the angle of contact from a line on the surface of the glass to the tangent at the point of contact through the liquid (as shown in the figure).

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




