
Explain what you understand from the diagram:
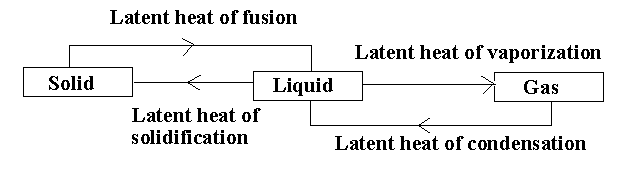

Answer
571.8k+ views
Hint:The transition taking place between the three states solid, liquid and gas involves a considerable amount of latent heat which is either absorbed during the process or released during the process.
Complete step by step answer:Latent heat is described as the energy released or absorbed when a compound changes its state. Latent heat is related with the enthalpy of change.
In the given diagram, there are different processes taking place.
The solid compound changes its state to liquid by the process of melting. The amount of heat acquired by the solid compound to transform into a liquid compound without increasing the temperature is called the latent heat of fusion.
The liquid compound changes its state to gas by the process of evaporation. The heat consumed or released when the liquid compound changes its state to gas at a constant temperature is known as latent heat of vapourization.
The liquid compound changes its state to solid by the process of freezing. The heat released when the liquid compound changes its state to solid at constant temperature is known as latent heat of solidification.
The gaseous compound changes its state to liquid by the process of condensation. The heat released when the gaseous compound changes to liquid is known as latent heat of condensation.
Note:The latent heat of condensation is reverse of latent heat of vapourization and the latent heat of solidification is reverse of latent heat of fusion. The latent heat is expressed in the form of joules or calories per mole.
Complete step by step answer:Latent heat is described as the energy released or absorbed when a compound changes its state. Latent heat is related with the enthalpy of change.

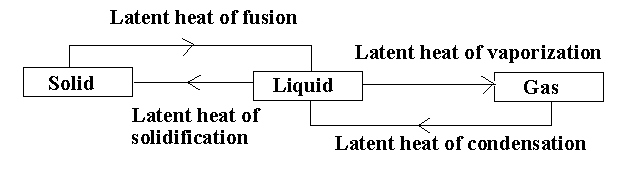
In the given diagram, there are different processes taking place.
The solid compound changes its state to liquid by the process of melting. The amount of heat acquired by the solid compound to transform into a liquid compound without increasing the temperature is called the latent heat of fusion.
The liquid compound changes its state to gas by the process of evaporation. The heat consumed or released when the liquid compound changes its state to gas at a constant temperature is known as latent heat of vapourization.
The liquid compound changes its state to solid by the process of freezing. The heat released when the liquid compound changes its state to solid at constant temperature is known as latent heat of solidification.
The gaseous compound changes its state to liquid by the process of condensation. The heat released when the gaseous compound changes to liquid is known as latent heat of condensation.
Note:The latent heat of condensation is reverse of latent heat of vapourization and the latent heat of solidification is reverse of latent heat of fusion. The latent heat is expressed in the form of joules or calories per mole.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




