
Explain this picture in your own words.

Answer
516.6k+ views
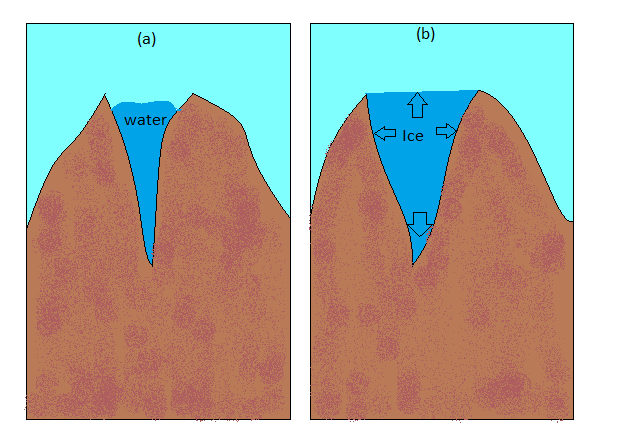
Hint: As we can clearly see the difference in the crevice or crack filled with water in both the images. Image ‘a’ has a crack filled with water in liquid phase whereas image ‘b’ simply represents ice filled in it with different length and width of crack. So we have to explain the reason behind the difference in the dimensions of crack or crevice of both the images.
Complete answer:
Let us discuss water and its varying properties in different states.
-Water: It is the most vital substance present on earth which is necessary for the survival of life. It shows quite different properties compared to other liquid substances. Its chemical formula is ${{H}_{2}}O$. It is a polar solvent and dissolves most of the chemical compounds easily.
-We know that when water molecules are in the liquid phase, hydrogen bonds between the molecules are continuously being formed and reformed in a random fashion. The average amount of hydrogen bonds formed by a single water molecule is 3.4 bonds at 25$^{\circ }C$.
-When water molecules freeze by losing energy, they do not vibrate or move vigorously which allows more stable hydrogen bonds to form between water molecules because there is less energy in order to break the bonds. Now the average hydrogen bonds per water molecule are 4 bonds.
-The hydrogen bonds are formed in an orderly, crystalline way that causes density to decrease as each water molecule is held away from its neighbors at an equal distance much alike to the length of the hydrogen bonds. This is the reason behind expansion of water as it freezes.
-So when the water in the crevice of image ‘a’ changed into ice by freezing, it simply expanded the crevice which brought changes in its overall dimension as shown in image ‘b’.
Note:
-Remember that the crystalline arrangement of water molecules is less dense than that of the molecules in liquid form due to which ice is less dense than the liquid water. This property can be easily noticed as icebergs float on huge water bodies.
Complete answer:
Let us discuss water and its varying properties in different states.
-Water: It is the most vital substance present on earth which is necessary for the survival of life. It shows quite different properties compared to other liquid substances. Its chemical formula is ${{H}_{2}}O$. It is a polar solvent and dissolves most of the chemical compounds easily.
-We know that when water molecules are in the liquid phase, hydrogen bonds between the molecules are continuously being formed and reformed in a random fashion. The average amount of hydrogen bonds formed by a single water molecule is 3.4 bonds at 25$^{\circ }C$.
-When water molecules freeze by losing energy, they do not vibrate or move vigorously which allows more stable hydrogen bonds to form between water molecules because there is less energy in order to break the bonds. Now the average hydrogen bonds per water molecule are 4 bonds.
-The hydrogen bonds are formed in an orderly, crystalline way that causes density to decrease as each water molecule is held away from its neighbors at an equal distance much alike to the length of the hydrogen bonds. This is the reason behind expansion of water as it freezes.
-So when the water in the crevice of image ‘a’ changed into ice by freezing, it simply expanded the crevice which brought changes in its overall dimension as shown in image ‘b’.
Note:
-Remember that the crystalline arrangement of water molecules is less dense than that of the molecules in liquid form due to which ice is less dense than the liquid water. This property can be easily noticed as icebergs float on huge water bodies.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




