
Explain the synthesis of ammonia occurs by Haber’s process.
Answer
587.7k+ views
Hint: In the synthesis of ammonia by Haber’s process two gases react at elevated temperatures and pressure to give ammonia as a final product. Both the gases are diatomic gases in which the gases have molecular weight of 2$gmmo{{l}^{-1}}$ and $28gmmo{{l}^{-1}}$.
Complete answer:
The detailed explanation of Haber’s process is as below.
- Haber process, also called the Haber–Bosch process. The inventors of this reaction were Fritz Haber and Carl Bosch. This reaction developed it in the first decade of the 20th century.
- This process converts atmospheric nitrogen gas (${{N}_{2}}$ ) to ammonia ($N{{H}_{3}}$ ) by a reaction with hydrogen (${{H}_{2}}$ ) using a metal catalyst under high temperatures and pressures:
The raw materials for the process are:
- Air which supplies the nitrogen to the system.
- Natural gas and water which supply the hydrogen and the energy needed to give heat to the reactants.
- Iron metal is used as a catalyst in this reaction.
Let us look at the reaction and diagram of Haber’s process.
\[{{N}_{2(g)}}+3{{H}_{2(g)}}\to 2N{{H}_{3(g)}}\,,\,\,\,\Delta {{H}^{\circ }}=-91.8\frac{kJ}{mole}\]
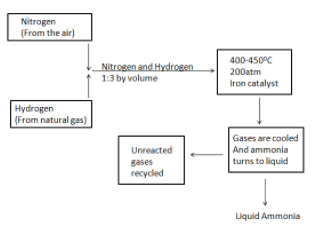
The above represented diagram is of production of liquid ammonia. Now, we will know about Haber’s process in detail.
- As shown in the diagram, in the Haber process we take nitrogen gas from the atmospheric air and combine it with hydrogen gas which is obtained from natural gas in the ratio 1:3 by volume.
- The gases are passed through four layers of catalyst which are called beds of catalyst and cooling takes place in each passing. This is done in order to maintain equilibrium constant. Unreacted gases are recycled from each step of the process.
- Normally, iron is used as a catalyst in the process and the whole procedure is conducted in a way that a temperature of around 400 – 450$^{\circ }C$ and a pressure of 150 – 200 atm is maintained thoroughly.
- The process also involves different steps like conversion of shift, removal of carbon dioxide and steam reforming.
- The ammonia gas is cooled down in the final stage of the process to form a liquid solution which is then collected and stored in containers.
Note:
Remember that nitrogen gas in the reaction is obtained by separating nitrogen from the air through liquefaction and hydrogen gas is obtained from natural gas by steam reforming. Note that any other catalyst than Iron does not give this reaction.
Complete answer:
The detailed explanation of Haber’s process is as below.
- Haber process, also called the Haber–Bosch process. The inventors of this reaction were Fritz Haber and Carl Bosch. This reaction developed it in the first decade of the 20th century.
- This process converts atmospheric nitrogen gas (${{N}_{2}}$ ) to ammonia ($N{{H}_{3}}$ ) by a reaction with hydrogen (${{H}_{2}}$ ) using a metal catalyst under high temperatures and pressures:
The raw materials for the process are:
- Air which supplies the nitrogen to the system.
- Natural gas and water which supply the hydrogen and the energy needed to give heat to the reactants.
- Iron metal is used as a catalyst in this reaction.
Let us look at the reaction and diagram of Haber’s process.
\[{{N}_{2(g)}}+3{{H}_{2(g)}}\to 2N{{H}_{3(g)}}\,,\,\,\,\Delta {{H}^{\circ }}=-91.8\frac{kJ}{mole}\]

The above represented diagram is of production of liquid ammonia. Now, we will know about Haber’s process in detail.
- As shown in the diagram, in the Haber process we take nitrogen gas from the atmospheric air and combine it with hydrogen gas which is obtained from natural gas in the ratio 1:3 by volume.
- The gases are passed through four layers of catalyst which are called beds of catalyst and cooling takes place in each passing. This is done in order to maintain equilibrium constant. Unreacted gases are recycled from each step of the process.
- Normally, iron is used as a catalyst in the process and the whole procedure is conducted in a way that a temperature of around 400 – 450$^{\circ }C$ and a pressure of 150 – 200 atm is maintained thoroughly.
- The process also involves different steps like conversion of shift, removal of carbon dioxide and steam reforming.
- The ammonia gas is cooled down in the final stage of the process to form a liquid solution which is then collected and stored in containers.
Note:
Remember that nitrogen gas in the reaction is obtained by separating nitrogen from the air through liquefaction and hydrogen gas is obtained from natural gas by steam reforming. Note that any other catalyst than Iron does not give this reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




