
Explain the structure of sulphur dioxide.
Answer
589.2k+ views
Hint: Calculate the oxidation number of sulphur in sulphur dioxide using algebraic calculations. It has an angular structure.
Complete step-by-step answer:
Sulphur dioxide is a binary compound, which means it consists of two elements and chemically, it is an oxide of sulphur with a chemical formula of$S{{O}_{2}}$.
We know that the structure of any compound has a central atom and to find it we can search for the atom which exhibits the highest oxidation number. In our case, we know that oxygen is more electronegative than sulphur and so the oxidation number of oxygen is$-2$. Taking the oxidation number of sulphur to be ‘x’, we form the following equation-
\[x+2\left( -2 \right)=0\]
We have equated the expression to ‘$0$’ because sulphur dioxide molecules are neutral in nature. By solving the above equation we get, ‘$+4$’ as the oxidation number of sulphur in sulphur dioxide. As it has the highest oxidation state, we take sulphur to be the central atom.
You must already know that the valency of oxygen is two and with only one central atom a double bond is to be expected. And indeed that is the case. The hybridization of sulphur is$s{{p}^{2}}$, which gives it a trigonal planar geometry. But the actual shape of the molecule is different because of the presence of lone pairs on sulphur.
But why are there lone pairs on sulphur? To answer this, let’s look again at the oxidation state of sulphur. We had calculated it to be$+4$; but there are six electrons in the valence shell of sulphur. That means there would be two electrons left which do not participate in bond formation. This lone pair is responsible for distortion in the shape of sulphur dioxide molecules. So, the bond angle is$119{}^\circ $in place of the actual bond angle of trigonal planar which is$180{}^\circ $.
The sulphur dioxide molecule thus has a bent shape just like water. It is shown as follows:
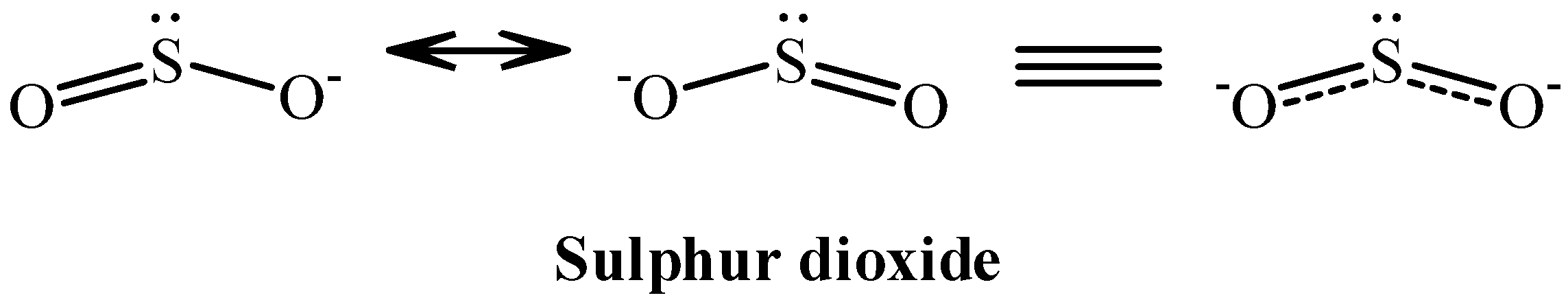
Note: As you can see, the structure of sulphur dioxide is actually the resonance hybrid between two canonical structures. The resonance hybrid is a dynamic structure, which means it exists and does not exist at the same time.
Complete step-by-step answer:
Sulphur dioxide is a binary compound, which means it consists of two elements and chemically, it is an oxide of sulphur with a chemical formula of$S{{O}_{2}}$.
We know that the structure of any compound has a central atom and to find it we can search for the atom which exhibits the highest oxidation number. In our case, we know that oxygen is more electronegative than sulphur and so the oxidation number of oxygen is$-2$. Taking the oxidation number of sulphur to be ‘x’, we form the following equation-
\[x+2\left( -2 \right)=0\]
We have equated the expression to ‘$0$’ because sulphur dioxide molecules are neutral in nature. By solving the above equation we get, ‘$+4$’ as the oxidation number of sulphur in sulphur dioxide. As it has the highest oxidation state, we take sulphur to be the central atom.
You must already know that the valency of oxygen is two and with only one central atom a double bond is to be expected. And indeed that is the case. The hybridization of sulphur is$s{{p}^{2}}$, which gives it a trigonal planar geometry. But the actual shape of the molecule is different because of the presence of lone pairs on sulphur.
But why are there lone pairs on sulphur? To answer this, let’s look again at the oxidation state of sulphur. We had calculated it to be$+4$; but there are six electrons in the valence shell of sulphur. That means there would be two electrons left which do not participate in bond formation. This lone pair is responsible for distortion in the shape of sulphur dioxide molecules. So, the bond angle is$119{}^\circ $in place of the actual bond angle of trigonal planar which is$180{}^\circ $.
The sulphur dioxide molecule thus has a bent shape just like water. It is shown as follows:

Note: As you can see, the structure of sulphur dioxide is actually the resonance hybrid between two canonical structures. The resonance hybrid is a dynamic structure, which means it exists and does not exist at the same time.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




