
Explain the purpose of staircase line. What type of elements are on either side of (along) the line?
Answer
493.2k+ views
Hint: The periodic table, commonly known as the periodic table of elements, is a tabular representation of chemical elements organised by atomic number, electron configuration, and recurrent chemical characteristics. The table's structure reveals periodic patterns. Metals are on the left and nonmetals are on the right in the table's seven rows, known as eras. The elements in the columns, referred to as groupings, have comparable chemical properties.
Complete Step By Step Answer:
On several depictions of the periodic table of the elements, the dividing line between metals and nonmetals can be found in various forms (see mini-example, right). The elements on the bottom left of the line have more metallic behaviour, whereas the elements on the upper right have more nonmetallic behaviour. The elements with the greatest critical temperature for respective groups (Li, Be, Al, Ge, Sb, Po) are barely below the line when shown as a conventional stair-step.
The amphoteric line, also known as the metal-nonmetal line, the metalloid line, the semimetal line, or the staircase, is a line that connects two metals. It's sometimes known as the Zintl boundary or the Zintl line, which is incorrect. Instead, the final two words allude to a vertical line that is occasionally drawn between groups 13 and 14. Laves named this specific line in 1941. It distinguishes components in group 13 from those in and to the right of group 14. The former frequently forms intermetallic compounds with electropositive metals, whereas the latter commonly forms salt-like compounds.
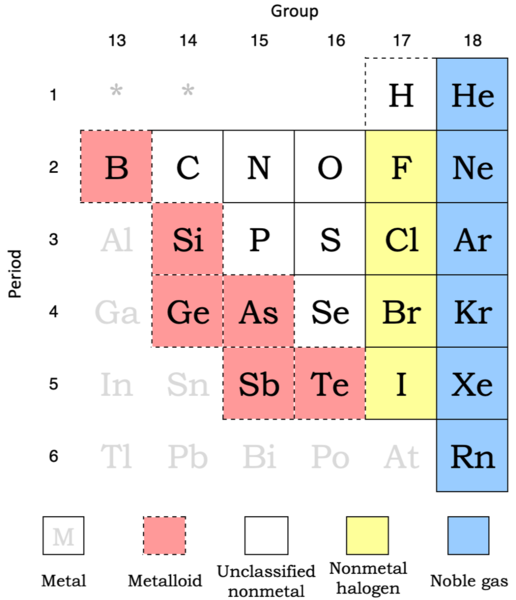
A metalloid is a chemical element with a majority of characteristics that fall between those of metals and those of nonmetals, or that are a combination of the two. There is no universally accepted definition of a metalloid, and there is no consensus on which elements are metalloids. Despite its lack of clarity, the word is still used in chemical literature.
Note:
Boron, silicon, germanium, arsenic, antimony, and tellurium are the six frequently recognised metalloids. Carbon, aluminium, selenium, polonium, and astatine are five of the least often categorised elements. All eleven elements are found in a diagonal area of the p-block running from boron at the upper left to astatine at the lower right on a conventional periodic table. Some periodic tables have a line between metals from nonmetals, and metalloids are often found around this line.
Complete Step By Step Answer:
On several depictions of the periodic table of the elements, the dividing line between metals and nonmetals can be found in various forms (see mini-example, right). The elements on the bottom left of the line have more metallic behaviour, whereas the elements on the upper right have more nonmetallic behaviour. The elements with the greatest critical temperature for respective groups (Li, Be, Al, Ge, Sb, Po) are barely below the line when shown as a conventional stair-step.
The amphoteric line, also known as the metal-nonmetal line, the metalloid line, the semimetal line, or the staircase, is a line that connects two metals. It's sometimes known as the Zintl boundary or the Zintl line, which is incorrect. Instead, the final two words allude to a vertical line that is occasionally drawn between groups 13 and 14. Laves named this specific line in 1941. It distinguishes components in group 13 from those in and to the right of group 14. The former frequently forms intermetallic compounds with electropositive metals, whereas the latter commonly forms salt-like compounds.

A metalloid is a chemical element with a majority of characteristics that fall between those of metals and those of nonmetals, or that are a combination of the two. There is no universally accepted definition of a metalloid, and there is no consensus on which elements are metalloids. Despite its lack of clarity, the word is still used in chemical literature.
Note:
Boron, silicon, germanium, arsenic, antimony, and tellurium are the six frequently recognised metalloids. Carbon, aluminium, selenium, polonium, and astatine are five of the least often categorised elements. All eleven elements are found in a diagonal area of the p-block running from boron at the upper left to astatine at the lower right on a conventional periodic table. Some periodic tables have a line between metals from nonmetals, and metalloids are often found around this line.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




