
Explain the important aspects of resonance with reference to the ${ CO }_{ 3 }^{ 2- }$ ion.
Answer
595.2k+ views
Hint: Resonance: It is defined as the transfer of electrons from multiple bonds or a lone pair of electrons from an atom to another atom or to an adjacent single covalent bond.
Complete step-by-step answer:
Resonance can be defined as the phenomenon of the existence of a molecule in many structures due to the delocalization of electrons. These structures are similar in energy, bonding, and nonbonding pairs of electrons. Only the distribution of electrons is different. In other words, if a molecule or an ion can be shown by ${ 2 }$ or more structures differing only in the distribution of electrons (but are otherwise the same in terms of number of electrons, energy, bonding atoms, etc), then the phenomenon is called resonance. These different structures of the molecule are called resonating, canonical, or contributing structures and none of them truly explain all the properties of the molecule or ion.
Thus a resonance structure is nothing but an alternate way of drawing a Lewis dot structure for a compound. There are many ways to draw a Lewis dot structure for some molecules that still satisfy the rules (for instance, satisfying the octet rule on each atom & having the correct total electron count).
Now let us consider drawing different resonating structures with the help of carbonate ion ${ CO }_{ 3 }^{ 2- }$. Resonating structures of this ion are represented as follows;
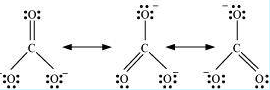
Figure: Resonating structures of carbonate ion
Here the central oxygen atom is bonded to three oxygen atoms. As these atoms are chemically identical, therefore any of these atoms can carry a negative charge or can be bonded to the carbon atoms by a double bond. In other words, the negative charge can reside on any of the oxygen atoms. Thus these structures differ only in the distribution of electrons but are otherwise the same. Hence, in each resonating structure, each separate oxygen atom will be bonded by a double bond, while the remaining two oxygen atoms will possess a negative charge.
Note: The possibility to make a mistake is that the negative charge persists on one oxygen atom but the atoms are identical, so any of these atoms can carry a negative charge.
Complete step-by-step answer:
Resonance can be defined as the phenomenon of the existence of a molecule in many structures due to the delocalization of electrons. These structures are similar in energy, bonding, and nonbonding pairs of electrons. Only the distribution of electrons is different. In other words, if a molecule or an ion can be shown by ${ 2 }$ or more structures differing only in the distribution of electrons (but are otherwise the same in terms of number of electrons, energy, bonding atoms, etc), then the phenomenon is called resonance. These different structures of the molecule are called resonating, canonical, or contributing structures and none of them truly explain all the properties of the molecule or ion.
Thus a resonance structure is nothing but an alternate way of drawing a Lewis dot structure for a compound. There are many ways to draw a Lewis dot structure for some molecules that still satisfy the rules (for instance, satisfying the octet rule on each atom & having the correct total electron count).
Now let us consider drawing different resonating structures with the help of carbonate ion ${ CO }_{ 3 }^{ 2- }$. Resonating structures of this ion are represented as follows;

Figure: Resonating structures of carbonate ion
Here the central oxygen atom is bonded to three oxygen atoms. As these atoms are chemically identical, therefore any of these atoms can carry a negative charge or can be bonded to the carbon atoms by a double bond. In other words, the negative charge can reside on any of the oxygen atoms. Thus these structures differ only in the distribution of electrons but are otherwise the same. Hence, in each resonating structure, each separate oxygen atom will be bonded by a double bond, while the remaining two oxygen atoms will possess a negative charge.
Note: The possibility to make a mistake is that the negative charge persists on one oxygen atom but the atoms are identical, so any of these atoms can carry a negative charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




