
Explain the hybridization of the central atom in $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$.
Answer
586.8k+ views
Hint: In$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$, the $\text{ Xe}$ atom is at the central atom. The two fluorine atoms are surrounding the $\text{ Xe}$ atom.$\text{ Xe}$ has 8 valence electrons. Two of these electrons are utilized in forming $\text{ Xe}-\text{F }$ bonds. Therefore, the total bond pairs are 2 and lone pairs are 3. Thus, $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ has the 5 hybrid orbitals. These 5 hybrid orbitals arranged themselves in the space to minimize the repulsion between the bond pair and lone pairs to give a definite geometry to the molecule.
Complete step by step answer:
$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ is the molecular formula for the compound Xenon difluoride which is a powerful fluorinating and oxidizing agent. \[\text{Xe}{{\text{F}}_{4}}\] or xenon tetrafluoride and \[\text{Xe}{{\text{F}}_{6}}\] or xenon hexafluoride are compounds other than $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$, out of which $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ is the most stable compound.
$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ appears as a white crystalline solid and is used for fluorinating purposes in electrochemical procedures and laboratories. It also has a typical nauseating odor and is easily decomposed when it comes in contact with vapor or light.
The electronic configuration of $\text{ Xe}$ is as follows:
$\text{ Xe = }\left[ \text{Kr} \right]\text{ 4}{{\text{d}}^{\text{10}}}\text{ 5}{{\text{s}}^{\text{2}}}\text{ 5}{{\text{p}}^{\text{6}}}$
The valence shell contains the 8 electrons which are accommodated on the atom.
The Lewis structure of $\text{Xe}{{\text{F}}_{\text{2}}}$ is given as follows.
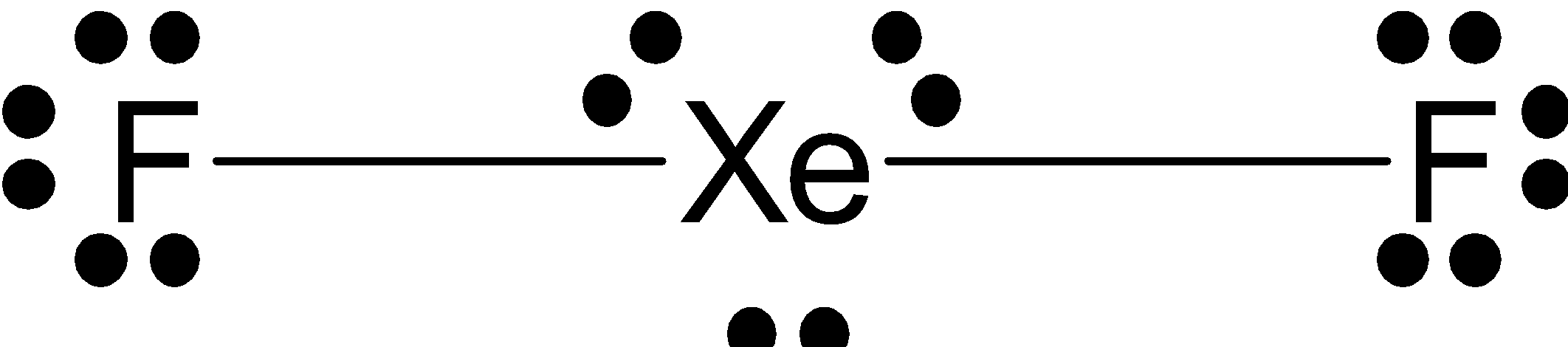
Lewis structure is based on the octet rule which states that every molecule must have 8 electrons in its outer shell of an atom to attain stability and if there are more electrons than that the compound must donate that electron, whereas if there are less electrons, the compound must accept electrons from other molecules to attain stability.
In case of$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$,
\[\begin{align}
& \text{number of valence electrons in Xe}{{\text{F}}_{\text{2}}}\text{ = no}\text{. of Valence Electrons in Xe }+\text{ 2}\times \text{(no}\text{. of Valence Electrons in F)} \\
& \text{number of valence electrons in Xe}{{\text{F}}_{\text{2}}}\text{ }=\text{ 8 + 2}\times \text{(7)} \\
& \therefore \text{number of valence electrons in Xe}{{\text{F}}_{\text{2}}}\text{ }=\text{ 22 }{{\text{e}}^{-\text{ }}}\text{ } \\
\end{align}\]
Hybridization of a given molecule is important for understanding the geometry of the molecule during formation of bonds. Two or more orbitals with various energy levels might get combined and make hybrid orbitals.
In case of $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$, the outer shell of Xenon has 8 electrons out of which two electrons participate in Bond formation. The ground state of Xenon has 8 electrons arranged in \[{{\text{s}}^{\text{2}}}\text{ }{{\text{p}}^{\text{6}}}\] orbitals while in $\text{Xe}{{\text{F}}_{\text{2}}}$, the $\text{ Xe}$ molecule has an excited state. This arrangement of electrons in xenon changes to \[{{\text{s}}^{\text{2}}}\text{ }{{\text{p}}^{\text{5}}}\text{ }{{\text{d}}^{\text{1}}}\] consisting of two unpaired electrons.
Therefore, the hybridization of the central atom $\text{ Xe}$ is \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\]. The \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\] hybridization of $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ molecule is shown below.
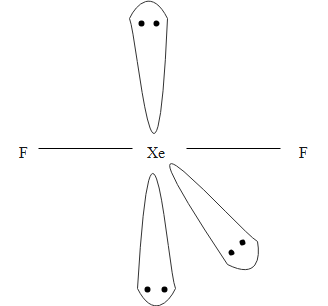
Note: The Lewis structure of any compound is crucial for knowing all physical and chemical properties of that particular compound Lewis structures are basically a pictorial representation of all the electrons participating in the formation of bonds. This structure also helps in understanding the charges on the molecules of that compound, the electrons participating in Bond formation as well as the ones that do not participate in bond formation as a whole, known as valence electrons.
Complete step by step answer:
$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ is the molecular formula for the compound Xenon difluoride which is a powerful fluorinating and oxidizing agent. \[\text{Xe}{{\text{F}}_{4}}\] or xenon tetrafluoride and \[\text{Xe}{{\text{F}}_{6}}\] or xenon hexafluoride are compounds other than $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$, out of which $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ is the most stable compound.
$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ appears as a white crystalline solid and is used for fluorinating purposes in electrochemical procedures and laboratories. It also has a typical nauseating odor and is easily decomposed when it comes in contact with vapor or light.
The electronic configuration of $\text{ Xe}$ is as follows:
$\text{ Xe = }\left[ \text{Kr} \right]\text{ 4}{{\text{d}}^{\text{10}}}\text{ 5}{{\text{s}}^{\text{2}}}\text{ 5}{{\text{p}}^{\text{6}}}$
The valence shell contains the 8 electrons which are accommodated on the atom.
The Lewis structure of $\text{Xe}{{\text{F}}_{\text{2}}}$ is given as follows.
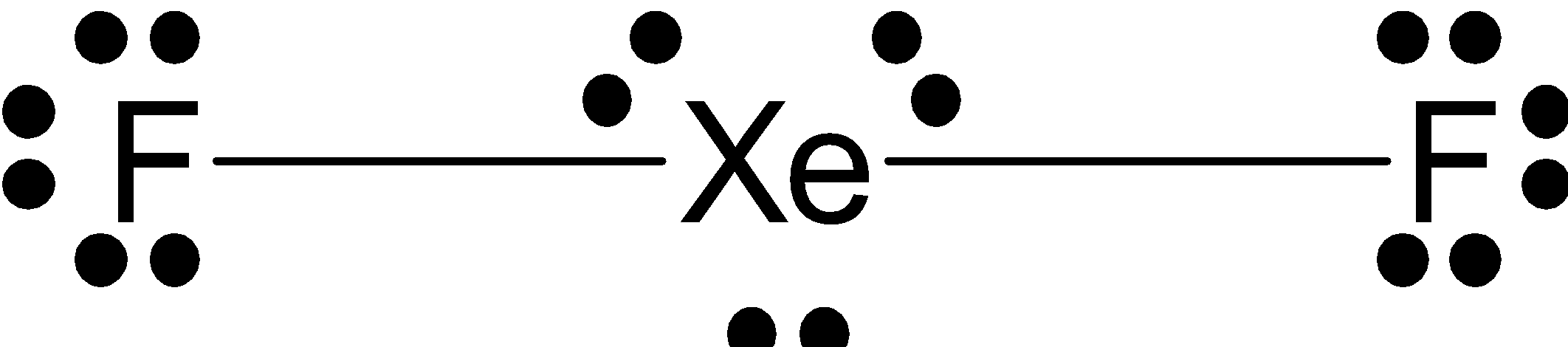
Lewis structure is based on the octet rule which states that every molecule must have 8 electrons in its outer shell of an atom to attain stability and if there are more electrons than that the compound must donate that electron, whereas if there are less electrons, the compound must accept electrons from other molecules to attain stability.
In case of$\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$,
\[\begin{align}
& \text{number of valence electrons in Xe}{{\text{F}}_{\text{2}}}\text{ = no}\text{. of Valence Electrons in Xe }+\text{ 2}\times \text{(no}\text{. of Valence Electrons in F)} \\
& \text{number of valence electrons in Xe}{{\text{F}}_{\text{2}}}\text{ }=\text{ 8 + 2}\times \text{(7)} \\
& \therefore \text{number of valence electrons in Xe}{{\text{F}}_{\text{2}}}\text{ }=\text{ 22 }{{\text{e}}^{-\text{ }}}\text{ } \\
\end{align}\]
Hybridization of a given molecule is important for understanding the geometry of the molecule during formation of bonds. Two or more orbitals with various energy levels might get combined and make hybrid orbitals.
In case of $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$, the outer shell of Xenon has 8 electrons out of which two electrons participate in Bond formation. The ground state of Xenon has 8 electrons arranged in \[{{\text{s}}^{\text{2}}}\text{ }{{\text{p}}^{\text{6}}}\] orbitals while in $\text{Xe}{{\text{F}}_{\text{2}}}$, the $\text{ Xe}$ molecule has an excited state. This arrangement of electrons in xenon changes to \[{{\text{s}}^{\text{2}}}\text{ }{{\text{p}}^{\text{5}}}\text{ }{{\text{d}}^{\text{1}}}\] consisting of two unpaired electrons.
Therefore, the hybridization of the central atom $\text{ Xe}$ is \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\]. The \[\text{s}{{\text{p}}^{\text{3}}}\text{d}\] hybridization of $\text{Xe}{{\text{F}}_{\text{2}}}\text{ }$ molecule is shown below.

Note: The Lewis structure of any compound is crucial for knowing all physical and chemical properties of that particular compound Lewis structures are basically a pictorial representation of all the electrons participating in the formation of bonds. This structure also helps in understanding the charges on the molecules of that compound, the electrons participating in Bond formation as well as the ones that do not participate in bond formation as a whole, known as valence electrons.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Class 11 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




