
Explain the hybridization of central atom in $SF_6.$
Answer
579k+ views
Hint: Hybridization is the concept which states that the existing orbitals of the atoms combine together to form new orbitals. These new orbitals have only unpaired electrons. . These new orbitals also influence the molecular structure as well as the bonding property of the atom.
Complete step by step answer:
Let’s start with discussing the concept of hybridization to understand the question better. Hybridization is the concept which states that the existing orbitals of the atoms combine together to form new orbitals. These new orbitals are known as the hybridized orbitals. These new orbitals also influence the molecular structure as well as the bonding property of the atom.
Coming back to the question, we are given the molecule $SF_6.$ and we need to find out the hybridization of the central atom. The central atom in the molecule $SF_6.$ is atom S which is sulphur.
When looking at the ground configuration of the central atom sulphur we get $3s^2 3p^4$, since the number of bonds forming is 6 which means that the orbitals should have 6 unpaired electrons. During hybridization the paired electrons are removed from their subshell and are placed in the new non occupied subshell. In case of Sulphur two electrons, one each from 3s and 3p orbital is placed inside the 3d orbital. This gives the hybridization of sp3d2 as one electron is in s orbital, 3 electron in p orbital and 2 electron in d orbital.
Therefore, the answer to this question is $sp^3d^2$.
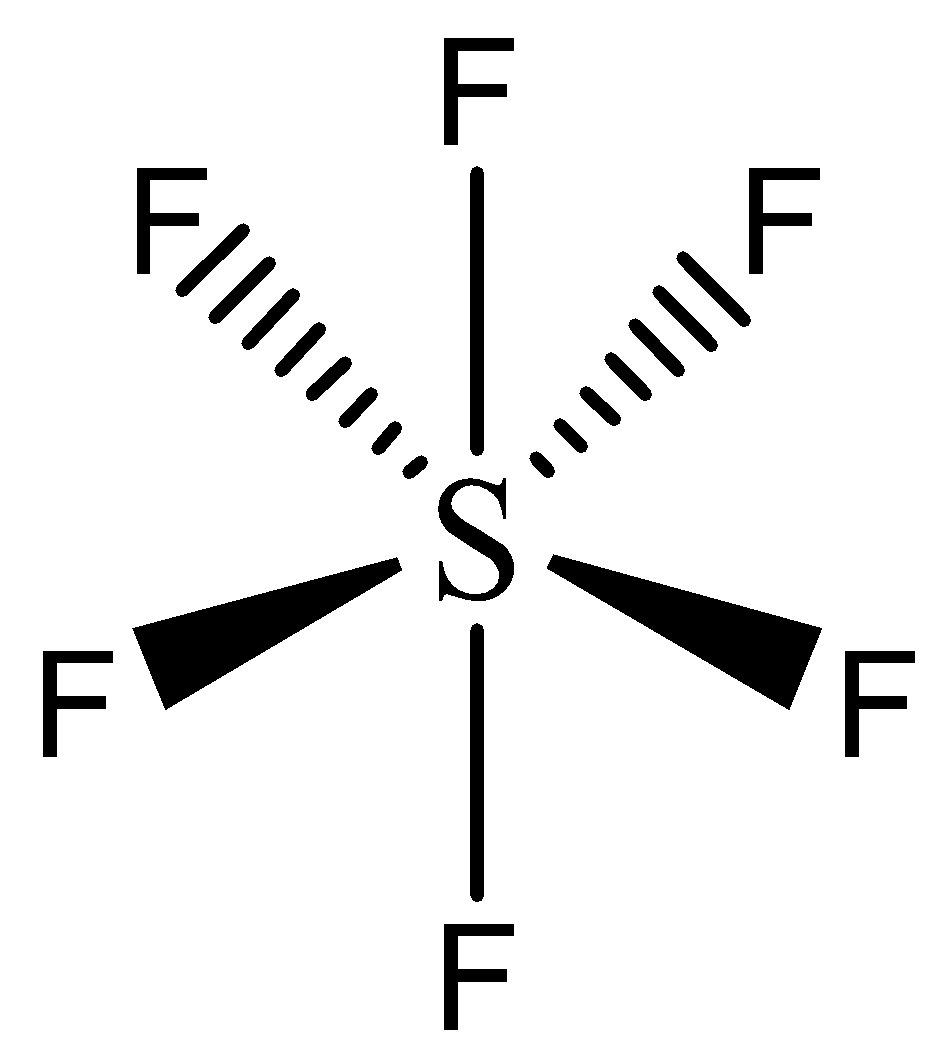
The structure of $SF_6.$ is given by,
Note:
As we know that the concept of hybridization is really important as it predicts the molecular geometry of the molecule within a reasonable approximation. It is important to know the geometry of the molecule as it helps in understanding the physical properties of the molecule. It also helps us understand the properties such as dipole moment, which is also a physical property.
Complete step by step answer:
Let’s start with discussing the concept of hybridization to understand the question better. Hybridization is the concept which states that the existing orbitals of the atoms combine together to form new orbitals. These new orbitals are known as the hybridized orbitals. These new orbitals also influence the molecular structure as well as the bonding property of the atom.
Coming back to the question, we are given the molecule $SF_6.$ and we need to find out the hybridization of the central atom. The central atom in the molecule $SF_6.$ is atom S which is sulphur.
When looking at the ground configuration of the central atom sulphur we get $3s^2 3p^4$, since the number of bonds forming is 6 which means that the orbitals should have 6 unpaired electrons. During hybridization the paired electrons are removed from their subshell and are placed in the new non occupied subshell. In case of Sulphur two electrons, one each from 3s and 3p orbital is placed inside the 3d orbital. This gives the hybridization of sp3d2 as one electron is in s orbital, 3 electron in p orbital and 2 electron in d orbital.
Therefore, the answer to this question is $sp^3d^2$.
The structure of $SF_6.$ is given by,

Note:
As we know that the concept of hybridization is really important as it predicts the molecular geometry of the molecule within a reasonable approximation. It is important to know the geometry of the molecule as it helps in understanding the physical properties of the molecule. It also helps us understand the properties such as dipole moment, which is also a physical property.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




