
Explain the formation of a triple bond with a diagram.
Answer
583.5k+ views
Hint: A triple bond is a covalent bond formed by sharing three pairs of electrons. Both the sigma bond and pi-bond are the two types of covalent bonding seen in the formation of multiple bonds.
Complete step by step solution:
A triple bond is made of three bond pairs, that is, three pairs of shared electrons.
Taking an example of the acetylene molecule, having a triple bond between the two-carbon atom, takes place as follows:
-The carbon atom has configuration $2{{s}^{2}}2{{p}^{2}}$, having four valence electrons in its ground state. In the excited state, one of the s-orbital electrons moves to the p-orbital.
The s-orbital being non-directional is on the internuclear axis (that is, Z-axis, a line which connects the centre of the nucleus of the two carbon atoms) and the ${{p}_{z}}$orbitals of the carbon is also directed along the internuclear axis.
-Thus, the two orbitals undergo overlapping to form the two $sp$ hybrid orbitals on the carbon atom, having one electron each. One of the $sp$hybrid orbital shares its electron forming a covalent bond with the $sp$hybrid orbital on the adjacent carbon atom, through end-to-end overlapping. Thus, a sigma bond is formed.
The other $sp$ hybrid orbital forms a bond with the s-orbital of the hydrogen atom.
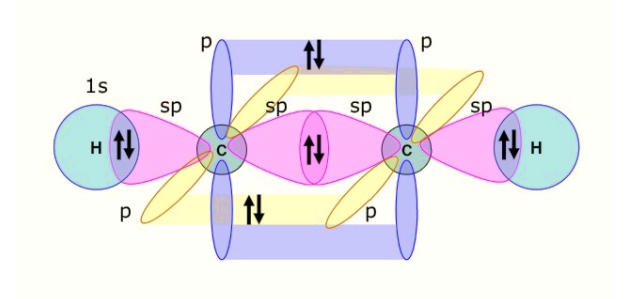
-We are left with the ${{p}_{x}}\,and\,{{p}_{y}}$ unhybridized orbitals with one electron each. These two are perpendicular to the internuclear axes. Thus, involving sideways overlapping of the ${{p}_{x}}\,and\,{{p}_{y}}$ orbitals with the ${{p}_{x}}\,and\,{{p}_{y}}$ of the adjacent carbon atom. Thus, forming two pi-bonds.
Therefore, a triple bond is formed in the acetylene molecule with two pi-bonds and one sigma bond.
Note: The hydrocarbons with triple bonds are called alkyne. The triple bond formation leads to an increase in bond strength, decrease in bond length, linear structure and increase in the melting and boiling point.
Complete step by step solution:
A triple bond is made of three bond pairs, that is, three pairs of shared electrons.
Taking an example of the acetylene molecule, having a triple bond between the two-carbon atom, takes place as follows:
-The carbon atom has configuration $2{{s}^{2}}2{{p}^{2}}$, having four valence electrons in its ground state. In the excited state, one of the s-orbital electrons moves to the p-orbital.
The s-orbital being non-directional is on the internuclear axis (that is, Z-axis, a line which connects the centre of the nucleus of the two carbon atoms) and the ${{p}_{z}}$orbitals of the carbon is also directed along the internuclear axis.
-Thus, the two orbitals undergo overlapping to form the two $sp$ hybrid orbitals on the carbon atom, having one electron each. One of the $sp$hybrid orbital shares its electron forming a covalent bond with the $sp$hybrid orbital on the adjacent carbon atom, through end-to-end overlapping. Thus, a sigma bond is formed.
The other $sp$ hybrid orbital forms a bond with the s-orbital of the hydrogen atom.

-We are left with the ${{p}_{x}}\,and\,{{p}_{y}}$ unhybridized orbitals with one electron each. These two are perpendicular to the internuclear axes. Thus, involving sideways overlapping of the ${{p}_{x}}\,and\,{{p}_{y}}$ orbitals with the ${{p}_{x}}\,and\,{{p}_{y}}$ of the adjacent carbon atom. Thus, forming two pi-bonds.
Therefore, a triple bond is formed in the acetylene molecule with two pi-bonds and one sigma bond.
Note: The hydrocarbons with triple bonds are called alkyne. The triple bond formation leads to an increase in bond strength, decrease in bond length, linear structure and increase in the melting and boiling point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




