
Explain the following: Tetravalency.
Answer
563.1k+ views
Hint: For solving this question, we need to understand the octet rule. As we know that a carbon atom has four electrons in its outermost valence shell. So, it needs four more electrons to complete its octet. For which it needs to form bonds with other atoms to get electrons and complete its octet.
Complete step by step answer:
As we know that the carbon is a periodic element present in the group $14$ and having atomic number of $6$ . The electronic configuration of carbon is represented by $1{s^2}2{s^2}2{p^2}$ which clearly shows that outermost four orbitals are half-filled. Thus it needs four electrons to form a stable compound.
So, the carbon atom forms four equivalent covalent bonds by sharing valence electrons with other atoms. This is known as tetravalency of carbon,(tetra means four). These four valences of carbon are directed towards four corners of a tetrahedron, and inclined to each other at an angle of $109.5^\circ $ .
We must know that the methane has a tetravalency nature with molecular formula of $C{H_4}$ and structure of methane is represented by ,
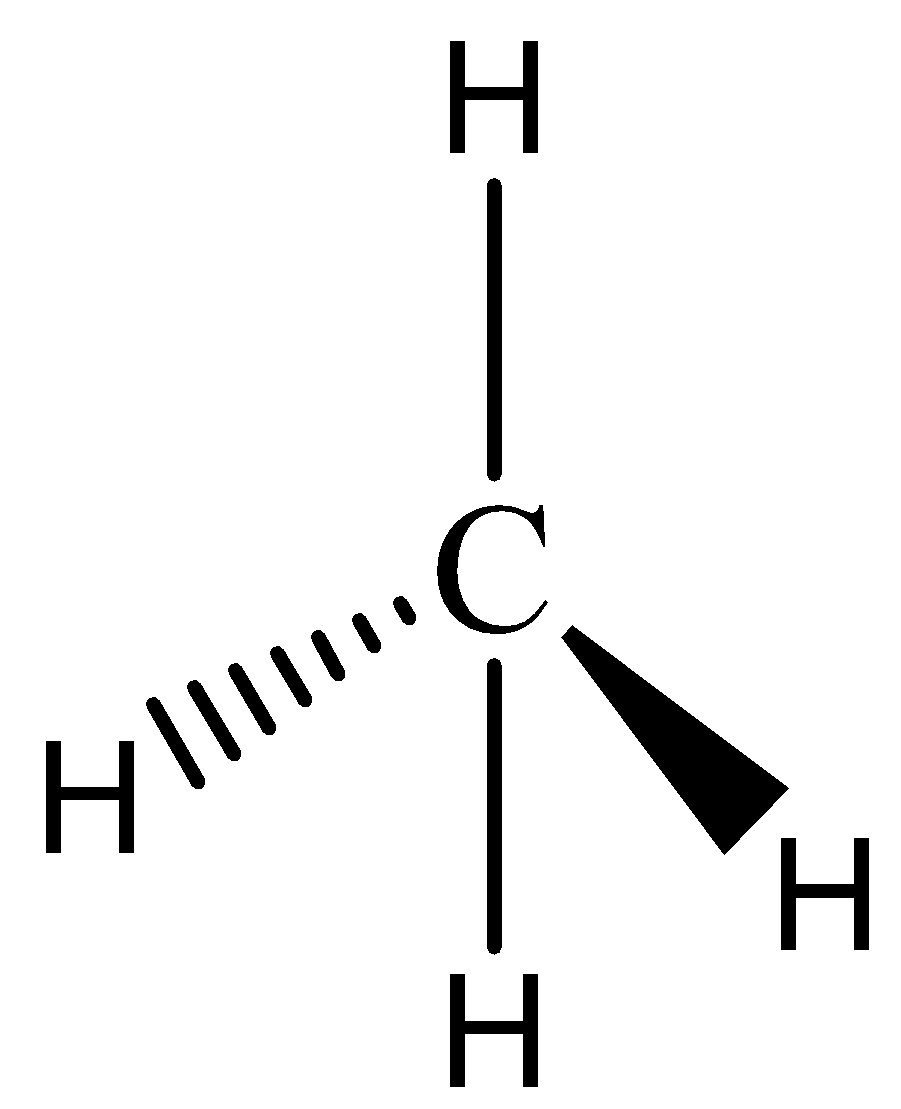
Here, we can see that the carbon atom is assumed to be the atomic center of the tetrahedron. The four valences of carbon are represented by four bonds that are around a carbon atom.
Also, we know that the total ionization energy required to remove four electrons from an atom is so high, that these elements do not form ionic compounds in their tetravalent state.
Note: We must remember that the no. of atoms present in an element which is replaced by another atom like hydrogen is known as valance. As we know that the noble gas present in group $18$ in the periodic table has a fulfilled valence electron thus they are more stable than the other elements. We have to remember that in a chemical reaction a compound is formed by sharing valence electrons of the two atoms.
Complete step by step answer:
As we know that the carbon is a periodic element present in the group $14$ and having atomic number of $6$ . The electronic configuration of carbon is represented by $1{s^2}2{s^2}2{p^2}$ which clearly shows that outermost four orbitals are half-filled. Thus it needs four electrons to form a stable compound.
So, the carbon atom forms four equivalent covalent bonds by sharing valence electrons with other atoms. This is known as tetravalency of carbon,(tetra means four). These four valences of carbon are directed towards four corners of a tetrahedron, and inclined to each other at an angle of $109.5^\circ $ .
We must know that the methane has a tetravalency nature with molecular formula of $C{H_4}$ and structure of methane is represented by ,

Here, we can see that the carbon atom is assumed to be the atomic center of the tetrahedron. The four valences of carbon are represented by four bonds that are around a carbon atom.
Also, we know that the total ionization energy required to remove four electrons from an atom is so high, that these elements do not form ionic compounds in their tetravalent state.
Note: We must remember that the no. of atoms present in an element which is replaced by another atom like hydrogen is known as valance. As we know that the noble gas present in group $18$ in the periodic table has a fulfilled valence electron thus they are more stable than the other elements. We have to remember that in a chemical reaction a compound is formed by sharing valence electrons of the two atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




