
Explain the bond formation in sodium chloride
Answer
590.4k+ views
Hint: Basically, sodium chloride is an ionic compound in which the sodium and chloride ions are in the ratio $1:1$ . The chemical formula of this compound is NaCl and it is commonly known as table salt or common salt or halite.
Complete step by step answer:
Sodium chloride is an ionic compound with the formula NaCl. So, basically sodium and chlorine respond together to generate a substance that is familiar to almost everybody known as sodium chloride or common salt. The reaction is as shown:
$2Na(s) + C{l_2}(g) \to 2NaCl(s)$
Moreover, in its aqueous state, it acts as a good conductor of electricity due to free movement of ions. It further has a melting point of ${801^ \circ }C$ and boiling point of ${1413^ \circ }C$ .Its structure is as shown:
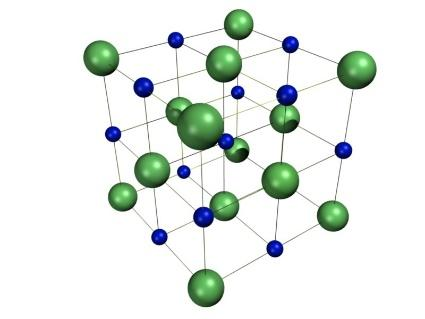
Now, the bond formed in this compound is basically an ionic bond. So, let’s see the electronic configuration of both the ions and then determine the structure. The electronic configuration of Na and Cl are:
Na- $1{s^2}2{s^2}2{p^6}3{s^1}$
Cl- $1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$
Now, Na is in excess of one electron to attain octet configuration whereas chlorine is deficient of one electron. So, one electron of Na atom is transferred to Cl atom and thus an ionic bond is formed due to the transfer of electrons. Therefore, NaCl has $N{a^ + }$ and $C{l^ - }$ atoms. Further, chlorine attains octet configuration by gaining one electron whereas sodium attains octet configuration by losing an electron.
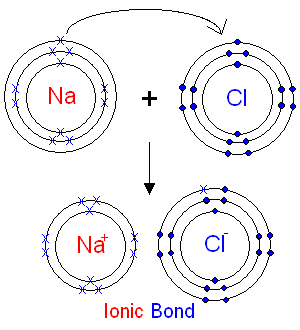
Note:Sodium chloride is widely used in food industries as a food preservative and as a flavor enhancer. Moreover, in cold countries, it is used to prevent the buildup of ice on roads, bridges etc. which is further important for safe driving. It is also used as a raw material in the industrial manufacturing of various chemicals such as sodium carbonate etc.
Complete step by step answer:
Sodium chloride is an ionic compound with the formula NaCl. So, basically sodium and chlorine respond together to generate a substance that is familiar to almost everybody known as sodium chloride or common salt. The reaction is as shown:
$2Na(s) + C{l_2}(g) \to 2NaCl(s)$
Moreover, in its aqueous state, it acts as a good conductor of electricity due to free movement of ions. It further has a melting point of ${801^ \circ }C$ and boiling point of ${1413^ \circ }C$ .Its structure is as shown:

Now, the bond formed in this compound is basically an ionic bond. So, let’s see the electronic configuration of both the ions and then determine the structure. The electronic configuration of Na and Cl are:
Na- $1{s^2}2{s^2}2{p^6}3{s^1}$
Cl- $1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$
Now, Na is in excess of one electron to attain octet configuration whereas chlorine is deficient of one electron. So, one electron of Na atom is transferred to Cl atom and thus an ionic bond is formed due to the transfer of electrons. Therefore, NaCl has $N{a^ + }$ and $C{l^ - }$ atoms. Further, chlorine attains octet configuration by gaining one electron whereas sodium attains octet configuration by losing an electron.

Note:Sodium chloride is widely used in food industries as a food preservative and as a flavor enhancer. Moreover, in cold countries, it is used to prevent the buildup of ice on roads, bridges etc. which is further important for safe driving. It is also used as a raw material in the industrial manufacturing of various chemicals such as sodium carbonate etc.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




