
Explain hybridization of the central atom in $\text{Xe}{{\text{F}}_{4}}$.
Answer
587.7k+ views
Hint: Find the hybridization of $\text{Xe}{{\text{F}}_{4}}$, using some rules and the formula X = quotient of division 1 + quotient when divided by 2. The hybridization of $\text{Xe}{{\text{F}}_{4}}$ is of the type $\text{A}{{\text{B}}_{4}}{{\text{L}}_{2}}$, where A is the central metal atom, B is neighbouring atoms and L is the lone pairs. Draw the structure and write the features of the structures.
Complete step by step answer:
First, we need to find the hybridization of $\text{Xe}{{\text{F}}_{4}}$:
(1) Count the valence electrons of elements present in the compound.
The valence electrons of Xe are 8 and of F are 7.
(2) Add the total valence electrons.
The valence electrons in the compound will be $\left[ 8+\left( 7\times 4 \right) \right]$ or 36 electrons.
(3) Divide the valence electrons by 8, and then divide the remainder by 2, until we get zero as a reminder.
So, $\frac{36}{8}$, quotient is 4 and remainder is 4. Further, $\frac{4}{2}$ is 2.
(4) The X = quotient of division 1+ quotient when divided by 2, will be equal to 4+2 or 6, X is the steric number of the compound.
The hybridization of $\text{Xe}{{\text{F}}_{4}}$ will be $\text{s}{{\text{p}}^{3}}{{\text{d}}^{2}}$.
Let us discuss the characteristics of $\text{Xe}{{\text{F}}_{4}}$ in terms of hybridization and structures:
(1) The hybridization in Xenon is $\text{s}{{\text{p}}^{3}}{{\text{d}}^{2}}$. The steric number of fluorine is 4, it is $\text{s}{{\text{p}}^{3}}$ hybridised.
(2) The central atom has 6 electron pairs in the structure out of which two are lone pair electrons and 4 are bond pairs between $\left( \text{Xe}-\text{F} \right)$. The structure of $\text{Xe}{{\text{F}}_{4}}$ is
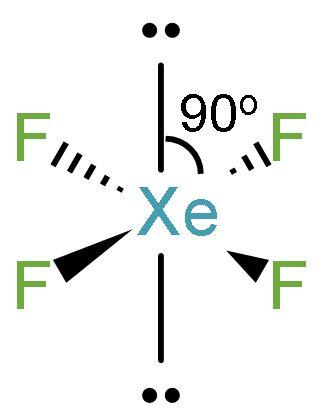
(3) $\text{Xe}{{\text{F}}_{4}}$ consists of two lone pair electrons. According to VSEPR theory, the bond pair electrons and lone pair electrons experience repulsion between them. There is ${{90}^{\text{o}}}$ angle between the bond pairs making it a stable compound. To minimize the repulsion, the lone pairs lie on the opposite sides of the molecule, which is at ${{180}^{\text{o}}}$ from each other.
(4) The lone pairs of Xenon lie in the perpendicular plane in an octahedral arrangement. Thus, the geometry of $\text{Xe}{{\text{F}}_{4}}$ is square planar.
Note: The main and important point to note while drawing the structure of any compound from its hybridization is that, it is not necessary that the type of arrangement of the compound is the same as its structure. Like, here, the arrangement of the compound is octahedral according to the hybridization but its structure is square planar. So, it is clear that the shape of the compound is decided by the arrangement of atoms or bonds not by the placing of the lone pairs.
Complete step by step answer:
First, we need to find the hybridization of $\text{Xe}{{\text{F}}_{4}}$:
(1) Count the valence electrons of elements present in the compound.
The valence electrons of Xe are 8 and of F are 7.
(2) Add the total valence electrons.
The valence electrons in the compound will be $\left[ 8+\left( 7\times 4 \right) \right]$ or 36 electrons.
(3) Divide the valence electrons by 8, and then divide the remainder by 2, until we get zero as a reminder.
So, $\frac{36}{8}$, quotient is 4 and remainder is 4. Further, $\frac{4}{2}$ is 2.
(4) The X = quotient of division 1+ quotient when divided by 2, will be equal to 4+2 or 6, X is the steric number of the compound.
The hybridization of $\text{Xe}{{\text{F}}_{4}}$ will be $\text{s}{{\text{p}}^{3}}{{\text{d}}^{2}}$.
Let us discuss the characteristics of $\text{Xe}{{\text{F}}_{4}}$ in terms of hybridization and structures:
(1) The hybridization in Xenon is $\text{s}{{\text{p}}^{3}}{{\text{d}}^{2}}$. The steric number of fluorine is 4, it is $\text{s}{{\text{p}}^{3}}$ hybridised.
(2) The central atom has 6 electron pairs in the structure out of which two are lone pair electrons and 4 are bond pairs between $\left( \text{Xe}-\text{F} \right)$. The structure of $\text{Xe}{{\text{F}}_{4}}$ is

(3) $\text{Xe}{{\text{F}}_{4}}$ consists of two lone pair electrons. According to VSEPR theory, the bond pair electrons and lone pair electrons experience repulsion between them. There is ${{90}^{\text{o}}}$ angle between the bond pairs making it a stable compound. To minimize the repulsion, the lone pairs lie on the opposite sides of the molecule, which is at ${{180}^{\text{o}}}$ from each other.
(4) The lone pairs of Xenon lie in the perpendicular plane in an octahedral arrangement. Thus, the geometry of $\text{Xe}{{\text{F}}_{4}}$ is square planar.
Note: The main and important point to note while drawing the structure of any compound from its hybridization is that, it is not necessary that the type of arrangement of the compound is the same as its structure. Like, here, the arrangement of the compound is octahedral according to the hybridization but its structure is square planar. So, it is clear that the shape of the compound is decided by the arrangement of atoms or bonds not by the placing of the lone pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




