
Explain hybridisation of $ I{F_7} $ and $ Cl{F_3} $ with electronic configuration and structure.
Answer
533.1k+ views
Hint: Hybridisation is the mixing of two pure orbitals of different energy to form a hybrid set of new orbitals of equivalent energy. We have to find the hybridisation following the set of rules according to the valence bond electron pair repulsion theory.
Complete Step By Step Answer:
We know that the hybridisation is mixing of two pure orbitals of different energy to form a hybrid set of new orbitals of equivalent energy.
We have to find the hybridisation of iodine heptafluoride . So first we find the hybridisation of the given molecule
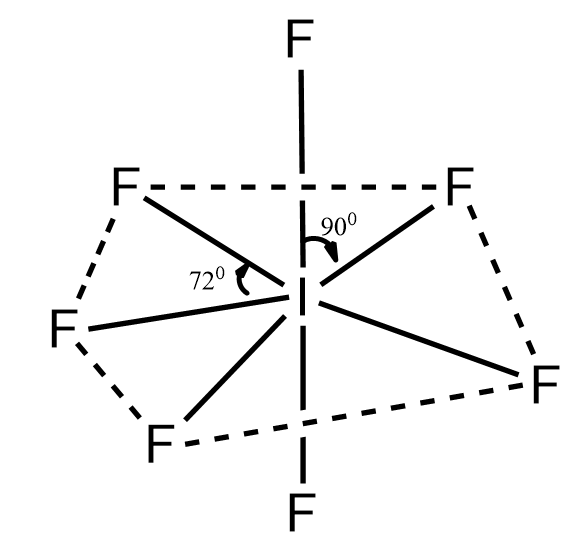
So in the molecule the number of valence electrons in a central metal atom is seven.
Number of bond pairs is equal to seven
So number of lone pairs would be $ 7 - 7 = 0 $
So the total pair is seven. So for seven pairs the hybridisation would be $ s{p^3}{d^3} $ and for the given hybridisation with zero lone pairs the structure would be pentagonal bipyramidal structure.
Now we have chlorine trifluoride molecules.
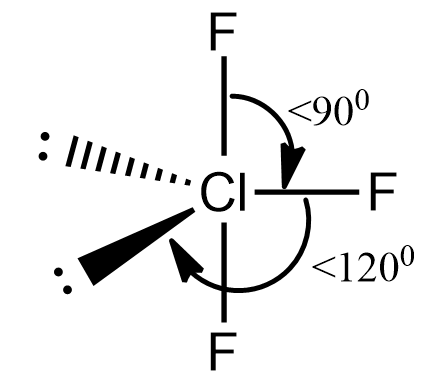
So in the molecule the number of valence electrons in a central metal atom is seven.
Number of bond pairs is equal to three
So the number of lone pairs would be $ 7 - 3 = 4 $ which in pairs means two lone pairs.
So the total pair is five which is the hybridisation $ s{p^3}d $ so the given hybridisation with two lone pairs would yield a t shape molecule.
Note:
Due to the presence of lone pairs the actual geometry changes which would be there if there was no lone pair present. The electron geometry and the shape varies for such cases. It also affects the bond angle and all such properties of the molecules.
Complete Step By Step Answer:
We know that the hybridisation is mixing of two pure orbitals of different energy to form a hybrid set of new orbitals of equivalent energy.
We have to find the hybridisation of iodine heptafluoride . So first we find the hybridisation of the given molecule

So in the molecule the number of valence electrons in a central metal atom is seven.
Number of bond pairs is equal to seven
So number of lone pairs would be $ 7 - 7 = 0 $
So the total pair is seven. So for seven pairs the hybridisation would be $ s{p^3}{d^3} $ and for the given hybridisation with zero lone pairs the structure would be pentagonal bipyramidal structure.
Now we have chlorine trifluoride molecules.

So in the molecule the number of valence electrons in a central metal atom is seven.
Number of bond pairs is equal to three
So the number of lone pairs would be $ 7 - 3 = 4 $ which in pairs means two lone pairs.
So the total pair is five which is the hybridisation $ s{p^3}d $ so the given hybridisation with two lone pairs would yield a t shape molecule.
Note:
Due to the presence of lone pairs the actual geometry changes which would be there if there was no lone pair present. The electron geometry and the shape varies for such cases. It also affects the bond angle and all such properties of the molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




