
Explain Haworth synthesis of naphthalene.
Answer
516.3k+ views
Hint: There are five steps involved in the formation of naphthalene by Haworth synthesis. These are Friedel craft acylation, Clemmenson’s reaction, heating the compound, Clemmenson reaction, and dehydrogenation. Two aromatic rings joined are known as naphthalene.
Complete answer:
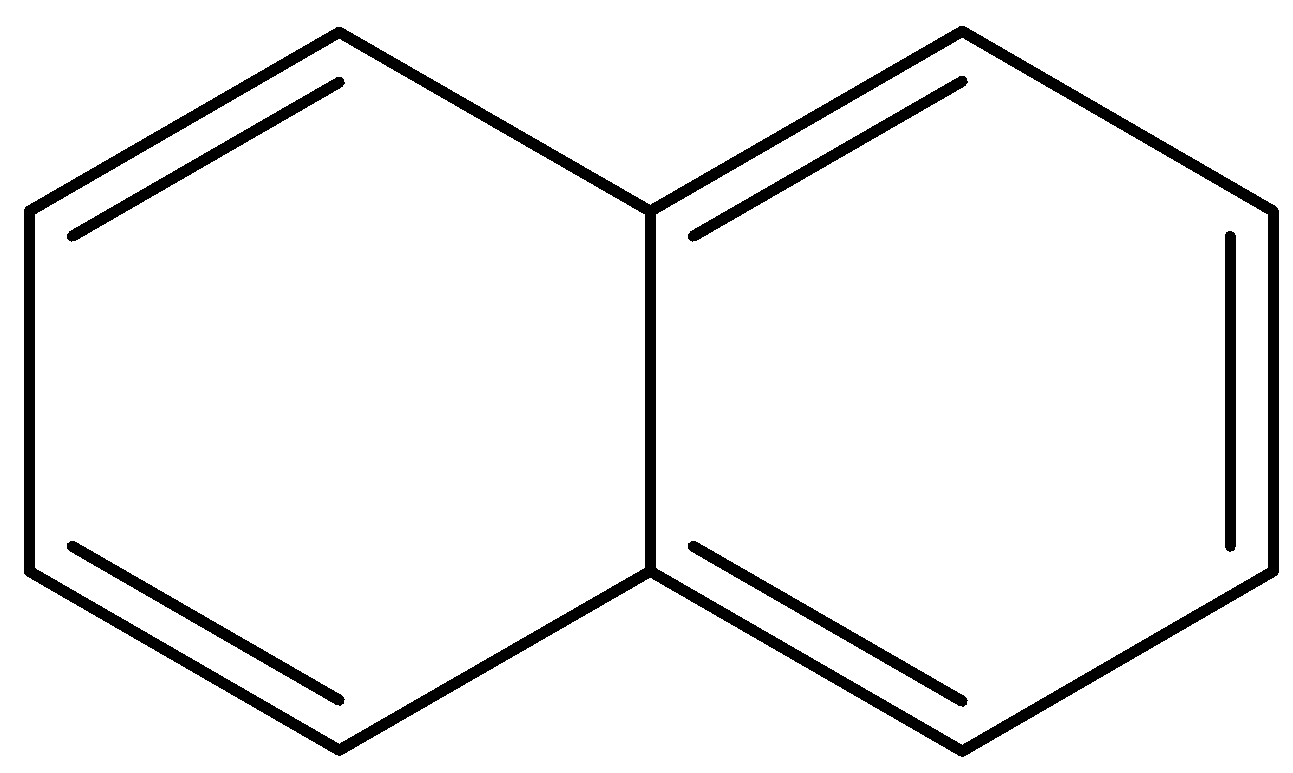
Naphthalene ${{C}_{10}}{{H}_{8}}$ is a simple, polycyclic aromatic hydrocarbon chemical molecule. It's a crystalline, white material with a distinctive smell. A fuse pair of benzene rings is the structure of Naphthalene. The constituent of traditional mothballs is best known.
The structure of naphthalene is given below:
The Haworth synthesis may be synthesized, the following stages are taken:
(i)- 3-benzoylpropionic acid is given via Benzene's craft acylation reaction with succinic anhydride.
(ii)- The second stage is the 3-benzoylpropionic acid reaction of Clemmenson, which results in 4-phenyl butanoic acid. Clemmensen reaction is observed in the presence of zinc amalgam and hydrochloric acid.
(iii)- The development of the ring structure of α-tetralene by elimination of the water molecule will result in this product being heated in the presence of strong sulphuric acid.
(iv)- Tetrahydronaphthalene is produced through the Clemmenson reaction of α-tetralene. Clemmensen reaction is observed in the presence of zinc amalgam and hydrochloric acid.
(v)- Tetrahydronaphthalene dehydrogenation produces naphthalene in the presence of selenium.
So, the reactions according to the steps are given below:
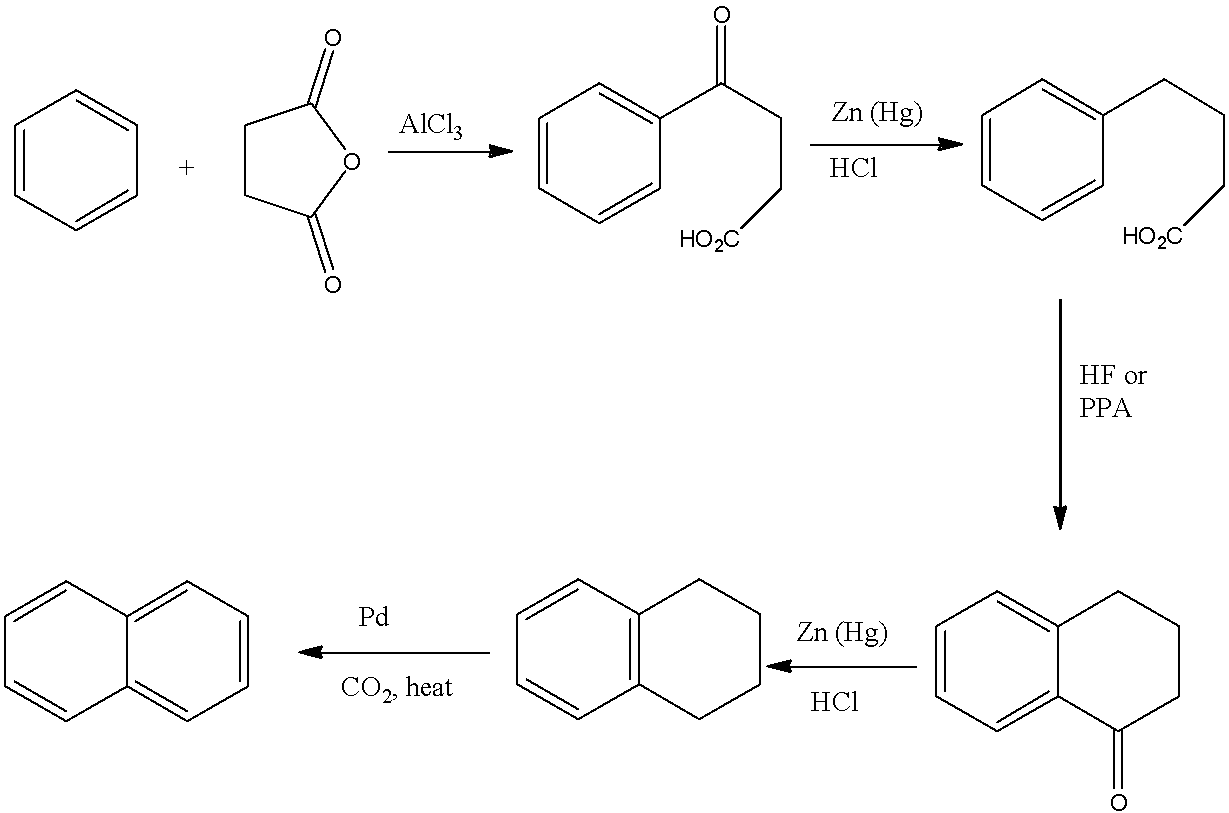
So, naphthalene is produced by Haworth synthesis by these steps.
Note:
Haworth synthesis is also used to produce other aromatic compounds like anthracene, phenanthrene, etc. Both anthracene and phenanthrene are aromatic compounds in which three benzene rings.
Complete answer:
Naphthalene ${{C}_{10}}{{H}_{8}}$ is a simple, polycyclic aromatic hydrocarbon chemical molecule. It's a crystalline, white material with a distinctive smell. A fuse pair of benzene rings is the structure of Naphthalene. The constituent of traditional mothballs is best known.
The structure of naphthalene is given below:

The Haworth synthesis may be synthesized, the following stages are taken:
(i)- 3-benzoylpropionic acid is given via Benzene's craft acylation reaction with succinic anhydride.
(ii)- The second stage is the 3-benzoylpropionic acid reaction of Clemmenson, which results in 4-phenyl butanoic acid. Clemmensen reaction is observed in the presence of zinc amalgam and hydrochloric acid.
(iii)- The development of the ring structure of α-tetralene by elimination of the water molecule will result in this product being heated in the presence of strong sulphuric acid.
(iv)- Tetrahydronaphthalene is produced through the Clemmenson reaction of α-tetralene. Clemmensen reaction is observed in the presence of zinc amalgam and hydrochloric acid.
(v)- Tetrahydronaphthalene dehydrogenation produces naphthalene in the presence of selenium.
So, the reactions according to the steps are given below:

So, naphthalene is produced by Haworth synthesis by these steps.
Note:
Haworth synthesis is also used to produce other aromatic compounds like anthracene, phenanthrene, etc. Both anthracene and phenanthrene are aromatic compounds in which three benzene rings.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




