
Explain each of the following observations:
A) With the same d-orbital configuration $\left( {{{\text{d}}^{\text{4}}}} \right)$, ${\text{C}}{{\text{r}}^{2 + }}$ is a reducing agent while ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is an oxidising agent.
B) Actinoids exhibit a much larger number of oxidation states than lanthanoids.
C) There is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals.
Answer
581.7k+ views
Hint: Any substance that oxidizes the other substance and itself gets reduced by gaining electrons is known as oxidising agent. Any substance that reduces the other substance and itself gets oxidised by losing electrons is known as reducing agent.
Complete step by step answer:
A) The outer electronic configuration of ${\text{Cr}}$ is $3{d^5}\,4{s^1}$.
The outer electronic configuration of ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ is $3{d^4}\,4{s^0}$.
${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ is a reducing agent. A reducing agent reduces the other species and itself gets oxidised. Thus, ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ gets oxidised by losing electrons. ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ can lose one electron and attain ${{\text{d}}^{\text{3}}}$ configuration.

The ${{\text{t}}_{{\text{2g}}}}$ configuration is more stable. Thus, ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ is an oxidising agent.
The outer electronic configuration of ${\text{Mn}}$ is $3{d^5}\,4{s^2}$.
The outer electronic configuration of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is $3{d^4}\,4{s^0}$.
${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is an oxidising agent. A oxidising agent oxidises the other species and itself gets reduced. Thus, ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ gets reduced by gaining electrons. ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ can gain one electron because it has a vacant 3d orbital and it can accommodate one electron.
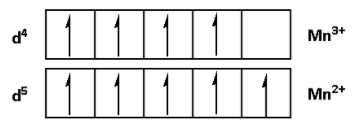
On gaining one electron the oxidation state of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ changes from ${\text{ + 3}}$ to ${\text{ + 2}}$.
${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ gains one electron and attains a stable configuration. Thus, ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ acts as an oxidising agent.
Thus, with the same d-orbital configuration $\left( {{{\text{d}}^{\text{4}}}} \right)$, ${\text{C}}{{\text{r}}^{2 + }}$ is a reducing agent while ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is an oxidising agent.
B) The similarities between actinoids and lanthanoids are as follows:
The size of atom: The size of actinoids and lanthanoids decreases across the series due to the actinoid contraction and lanthanoid contraction respectively.
The most common oxidation state of lanthanoid and actinoid is ${\text{ + 3}}$. Some actinoids can show oxidation states higher than ${\text{ + 3}}$ due to the energy difference between the orbitals being very less.
Thus, actinoids exhibit a much larger number of oxidation states than lanthanoids.
C) The atomic sizes of the elements of the first transition series are smaller than the atomic sizes of the elements in the second and the third transition series.
The atomic sizes of the elements of the third transition series are very similar to the elements of the second transition series. The reason for this is the lanthanoid contraction.
Thus, there is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals.
Note: The ${{\text{t}}_{{\text{2g}}}}$ configuration is more stable than the ${{\text{e}}_{\text{g}}}$ configuration. Thus, the ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ ion acts as an oxidising agent because it attains stable ${{\text{t}}_{{\text{2g}}}}$ configuration.
Complete step by step answer:
A) The outer electronic configuration of ${\text{Cr}}$ is $3{d^5}\,4{s^1}$.
The outer electronic configuration of ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ is $3{d^4}\,4{s^0}$.
${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ is a reducing agent. A reducing agent reduces the other species and itself gets oxidised. Thus, ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ gets oxidised by losing electrons. ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ can lose one electron and attain ${{\text{d}}^{\text{3}}}$ configuration.

The ${{\text{t}}_{{\text{2g}}}}$ configuration is more stable. Thus, ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ is an oxidising agent.
The outer electronic configuration of ${\text{Mn}}$ is $3{d^5}\,4{s^2}$.
The outer electronic configuration of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is $3{d^4}\,4{s^0}$.
${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is an oxidising agent. A oxidising agent oxidises the other species and itself gets reduced. Thus, ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ gets reduced by gaining electrons. ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ can gain one electron because it has a vacant 3d orbital and it can accommodate one electron.

On gaining one electron the oxidation state of ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ changes from ${\text{ + 3}}$ to ${\text{ + 2}}$.
${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ gains one electron and attains a stable configuration. Thus, ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ acts as an oxidising agent.
Thus, with the same d-orbital configuration $\left( {{{\text{d}}^{\text{4}}}} \right)$, ${\text{C}}{{\text{r}}^{2 + }}$ is a reducing agent while ${\text{M}}{{\text{n}}^{{\text{3 + }}}}$ is an oxidising agent.
B) The similarities between actinoids and lanthanoids are as follows:
The size of atom: The size of actinoids and lanthanoids decreases across the series due to the actinoid contraction and lanthanoid contraction respectively.
The most common oxidation state of lanthanoid and actinoid is ${\text{ + 3}}$. Some actinoids can show oxidation states higher than ${\text{ + 3}}$ due to the energy difference between the orbitals being very less.
Thus, actinoids exhibit a much larger number of oxidation states than lanthanoids.
C) The atomic sizes of the elements of the first transition series are smaller than the atomic sizes of the elements in the second and the third transition series.
The atomic sizes of the elements of the third transition series are very similar to the elements of the second transition series. The reason for this is the lanthanoid contraction.
Thus, there is hardly any increase in atomic size with increasing atomic numbers in a series of transition metals.
Note: The ${{\text{t}}_{{\text{2g}}}}$ configuration is more stable than the ${{\text{e}}_{\text{g}}}$ configuration. Thus, the ${\text{C}}{{\text{r}}^{{\text{2 + }}}}$ ion acts as an oxidising agent because it attains stable ${{\text{t}}_{{\text{2g}}}}$ configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




