
Explain ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}$ is an inner orbital complex whereas ${{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}$ is an outer orbital complex.
Answer
522.6k+ views
Hint: The ligand and the number of electrons in the valence orbitals of the metal are going to decide the complex is inner orbital or outer orbital complex. To form an inner orbital complex the ligand should be a strong ligand.
Complete answer:
- In the question it is asked to explain ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}$ is an inner orbital complex whereas ${{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}$ is an outer orbital complex.
- Coming to the complex ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}$ .
- In this complex cobalt in +3 oxidation.
- The electronic configuration of cobalt is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{7}}$ .
- The electronic configuration of $C{{o}^{3+}}$ is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{0}}3{{d}^{6}}$ .
- We can write the electron representation in $C{{o}^{3+}}$ as follows.
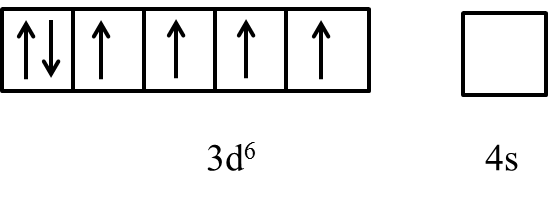
- In the presence of the ligand like ammonia the electrons in the 3d orbital are going to pair up and it is as follows.
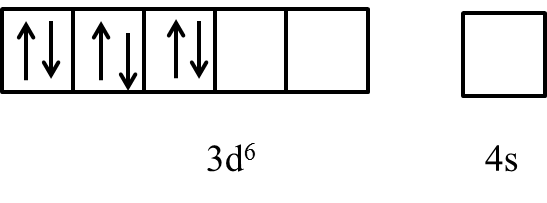
- After pairing the electron in the 3d orbital of the cobalt the last two orbitals are ready to participate in the formation of the bond with ligands and form an inner orbital complex with a hybridization of ${{d}^{2}}s{{p}^{3}}$ .
- Coming to ${{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}$ .
- In this complex Nickel is in +2 oxidation.
- The electronic configuration of Nickel is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{8}}$ .
- The electronic configuration of $N{{i}^{2+}}$ is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{0}}3{{d}^{8}}$ .
- We can write the electron representation in $N{{i}^{2+}}$ as follows.
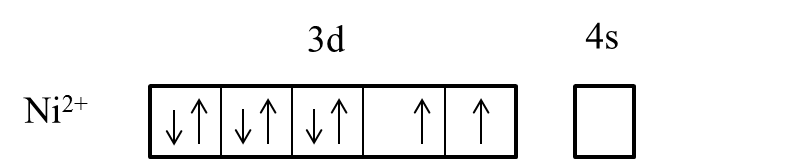
- In the presence of the ligand like ammonia the electrons in the 3d orbital are going to pair up and it is as follows.
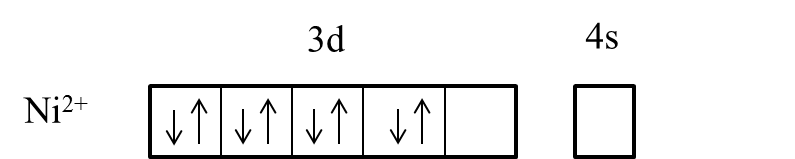
- After pairing the electrons in the 3d orbital of the Nickel, it has only one empty to participate in the hybridization.
- But it is not possible to participate only one 3d orbital in the hybridization, therefore nickel undergoes $s{{p}^{3}}{{d}^{2}}$ hybridization and forms an outer orbital complex with ligand ammonia.
Note:
There is an availability of d-orbitals in the process of hybridization to form inner orbital complexes. If there is no vacant 3d orbital in the metal then the metal undergoes outer orbital complex with the respective ligands.
Complete answer:
- In the question it is asked to explain ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}$ is an inner orbital complex whereas ${{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}$ is an outer orbital complex.
- Coming to the complex ${{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}$ .
- In this complex cobalt in +3 oxidation.
- The electronic configuration of cobalt is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{7}}$ .
- The electronic configuration of $C{{o}^{3+}}$ is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{0}}3{{d}^{6}}$ .
- We can write the electron representation in $C{{o}^{3+}}$ as follows.

- In the presence of the ligand like ammonia the electrons in the 3d orbital are going to pair up and it is as follows.

- After pairing the electron in the 3d orbital of the cobalt the last two orbitals are ready to participate in the formation of the bond with ligands and form an inner orbital complex with a hybridization of ${{d}^{2}}s{{p}^{3}}$ .
- Coming to ${{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}$ .
- In this complex Nickel is in +2 oxidation.
- The electronic configuration of Nickel is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{8}}$ .
- The electronic configuration of $N{{i}^{2+}}$ is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{0}}3{{d}^{8}}$ .
- We can write the electron representation in $N{{i}^{2+}}$ as follows.

- In the presence of the ligand like ammonia the electrons in the 3d orbital are going to pair up and it is as follows.

- After pairing the electrons in the 3d orbital of the Nickel, it has only one empty to participate in the hybridization.
- But it is not possible to participate only one 3d orbital in the hybridization, therefore nickel undergoes $s{{p}^{3}}{{d}^{2}}$ hybridization and forms an outer orbital complex with ligand ammonia.
Note:
There is an availability of d-orbitals in the process of hybridization to form inner orbital complexes. If there is no vacant 3d orbital in the metal then the metal undergoes outer orbital complex with the respective ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




