
Ethylidene chloride can be prepared by the reaction of \[HCl\] and:
A.Ethane
B.Ethylene
C.Acetylene
D.Ethylene glycol
Answer
544.5k+ views
Hint:In the question, we have given an incomplete reaction. So here we should know the preparation of Ethylidene chloride. So we can use the given options and then we will react to them \[HCl\]. If the product obtained from the reaction is Ethylidene chloride then that reaction will be considered as the preparation of Ethylidene chloride.
Complete step by step answer:
-First, we will understand the question properly. In the question, we have the product as Ethylidene chloride. We need to find the reactant which can react with \[HCl\] to give Ethylidene chloride.
-So, we will react to the compounds given in the question with Hydrochloric acid \[\left( {HCl} \right)\]. So let’s start with the reactions one by one.
A.First, we will react with Ethane with \[HCl\]. There is no reaction here as alkanes do not directly give a reaction \[HCl\].
B.Now we will react to Ethylene with \[HCl\]. It will undergo an electrophilic addition reaction so the product will be formed according to Markovnikov’s rule.
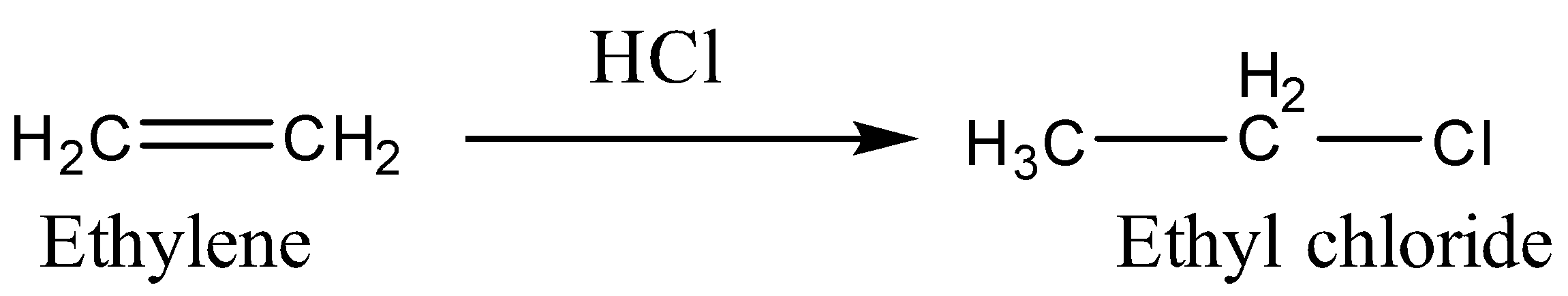
C.Now we will react Acetylene with \[HCl\]. The reaction will undergo electrophilic addition and the product will be formed according to Markovnikov’s rule.
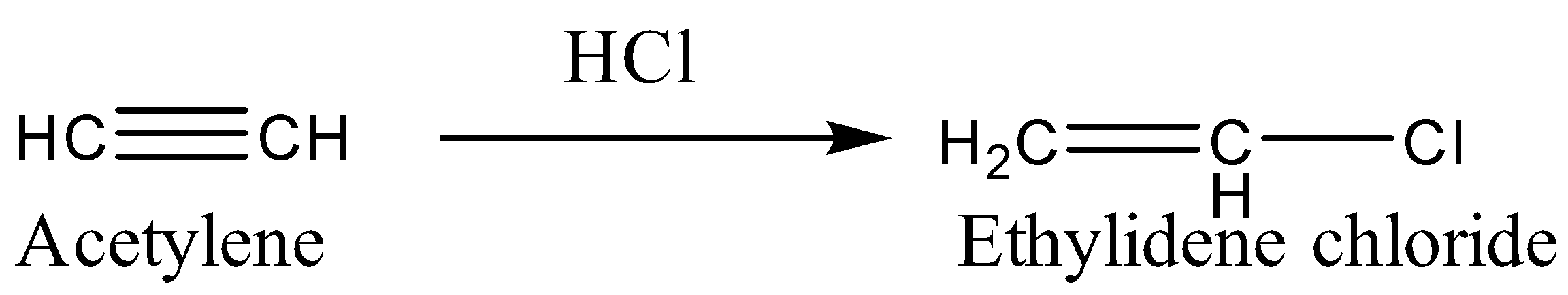
D. Ethylene glycol does not react \[HCl\] directly. It reacts with an aldehyde in the presence of dry \[HCl\] to give cyclic acetal.
-Therefore, from the above reactions, we can conclude that acetylene on reaction with \[HCl\] gives ethylidene chloride.
-The product is formed according to Markovnikov’s rule in which halogen gets attached to the carbon atom with less number of the hydrogen atom.
Hence, the correct option is (C).
Note:
If the question \[HCl\] is given in excess then the product of a reaction in which Acetylene reacts \[HCl\] will be Ethylidene dichloride. Due to excess \[HCl\], Ethylidene chloride will again undergo electrophilic addition and the product will be formed according to Markovnikov’s rule.
Complete step by step answer:
-First, we will understand the question properly. In the question, we have the product as Ethylidene chloride. We need to find the reactant which can react with \[HCl\] to give Ethylidene chloride.
-So, we will react to the compounds given in the question with Hydrochloric acid \[\left( {HCl} \right)\]. So let’s start with the reactions one by one.
A.First, we will react with Ethane with \[HCl\]. There is no reaction here as alkanes do not directly give a reaction \[HCl\].
B.Now we will react to Ethylene with \[HCl\]. It will undergo an electrophilic addition reaction so the product will be formed according to Markovnikov’s rule.

C.Now we will react Acetylene with \[HCl\]. The reaction will undergo electrophilic addition and the product will be formed according to Markovnikov’s rule.

D. Ethylene glycol does not react \[HCl\] directly. It reacts with an aldehyde in the presence of dry \[HCl\] to give cyclic acetal.
-Therefore, from the above reactions, we can conclude that acetylene on reaction with \[HCl\] gives ethylidene chloride.
-The product is formed according to Markovnikov’s rule in which halogen gets attached to the carbon atom with less number of the hydrogen atom.
Hence, the correct option is (C).
Note:
If the question \[HCl\] is given in excess then the product of a reaction in which Acetylene reacts \[HCl\] will be Ethylidene dichloride. Due to excess \[HCl\], Ethylidene chloride will again undergo electrophilic addition and the product will be formed according to Markovnikov’s rule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




