
Ethyl acetoacetate when reacts with one mole methyl magnesium iodide then product of reaction will be:
(a)-
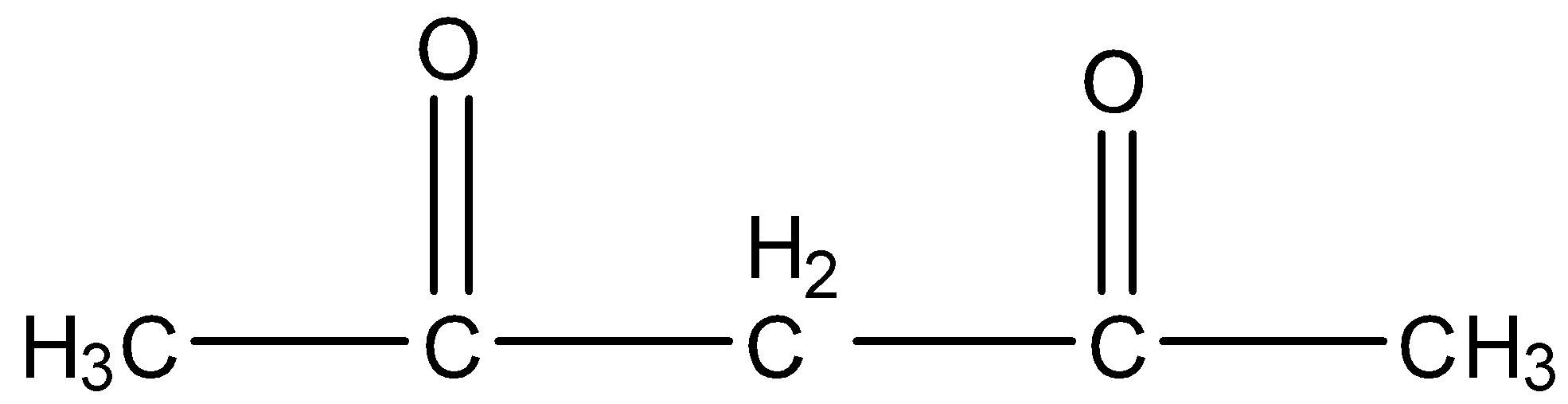
(b)-
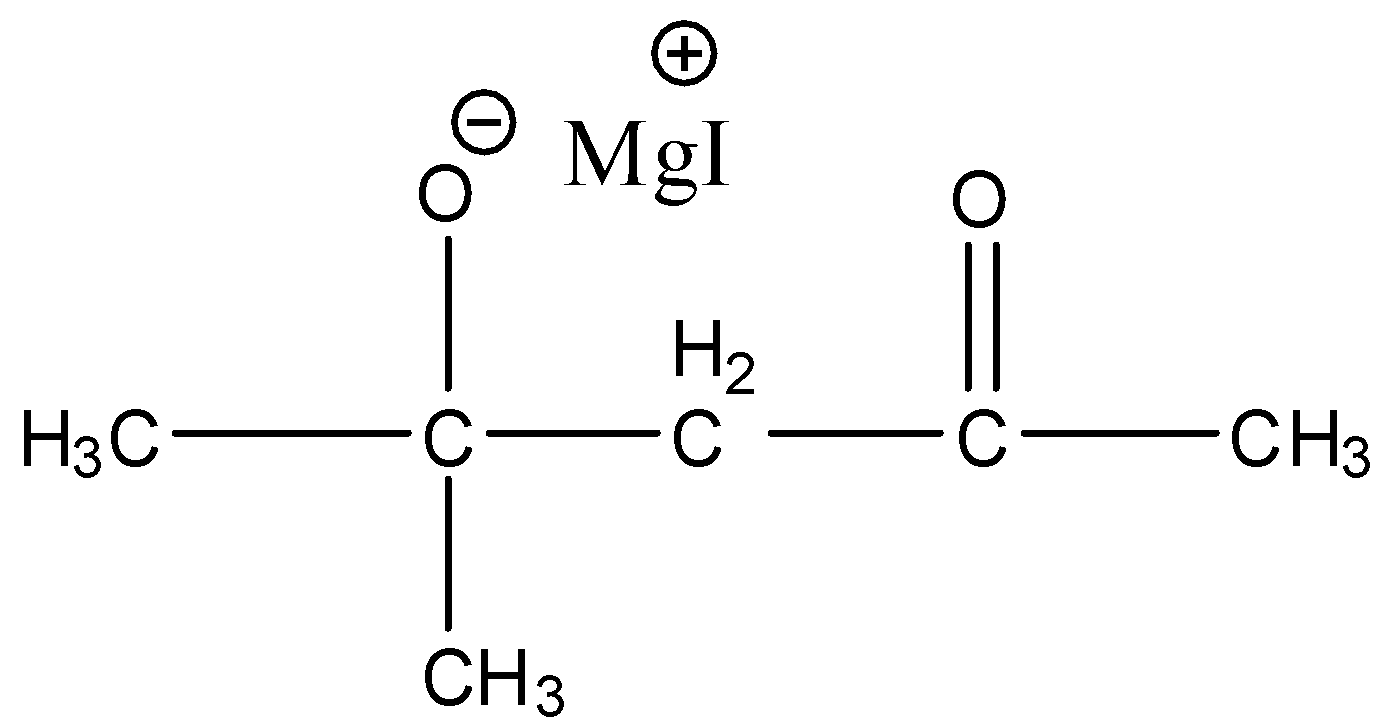
(c)-
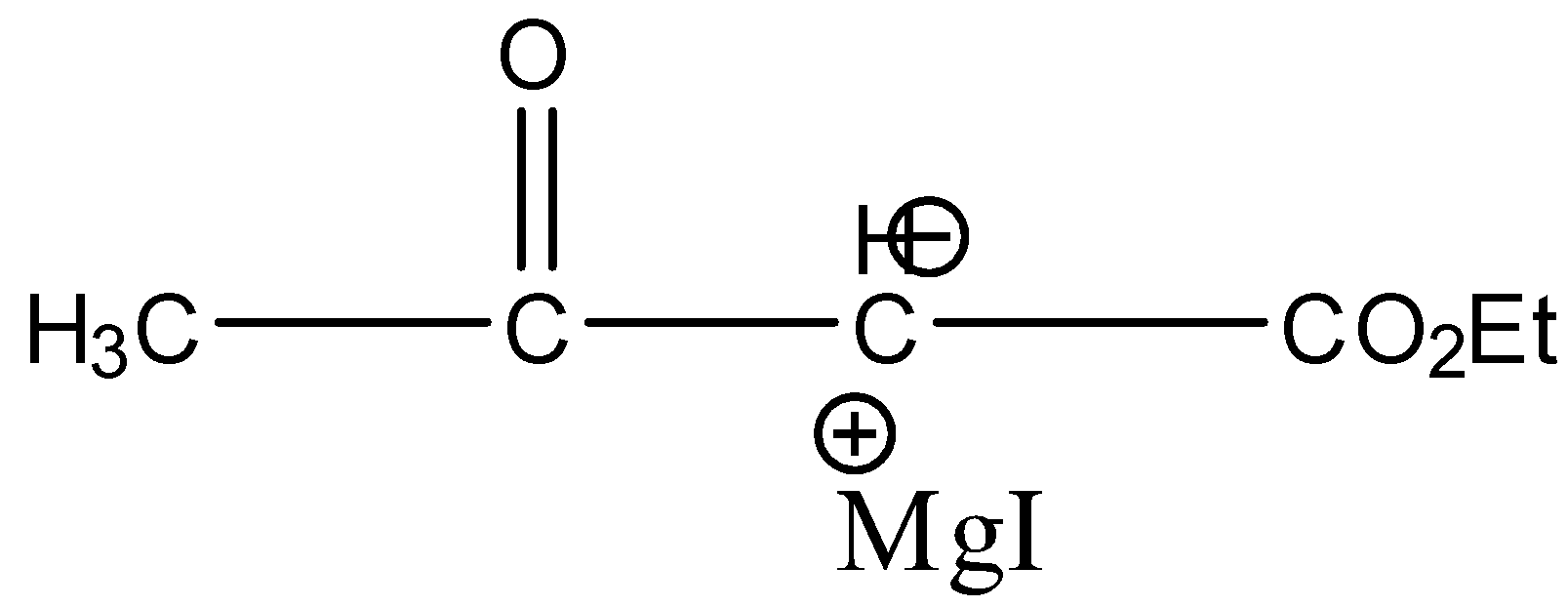
(d)-
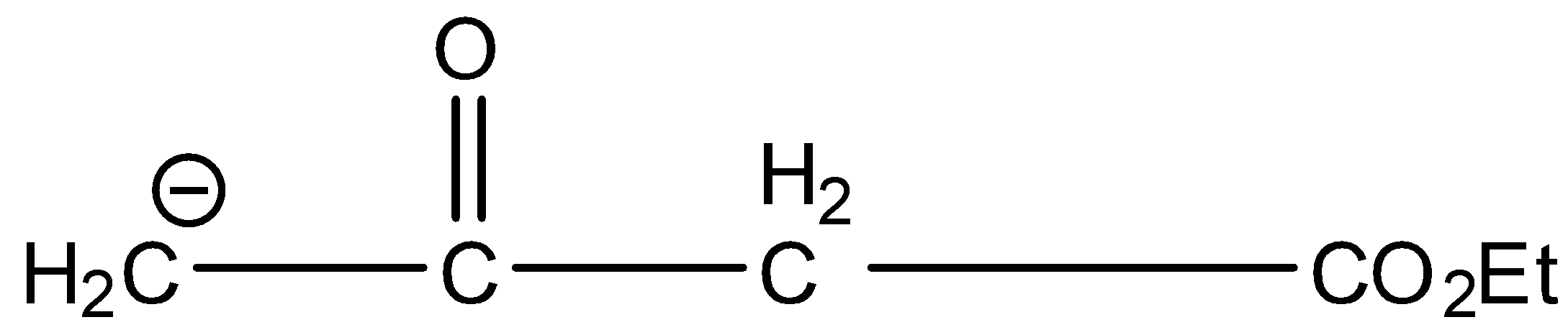




Answer
513.3k+ views
Hint: Ethyl acetoacetate is an organic compound in which there are six carbon atoms, the second carbon atom has the ketone group and the fourth carbon atom has the ester functional group. So, the carbon atom between the ketone and ester group is the acidic methyl group.
Complete answer:
Methyl magnesium iodide is a Grignard reagent in which the halogen atom is iodine and an alkyl group is a methyl group. The formula of methyl magnesium iodide is $C{{H}_{3}}MgI$.
The given compound in the question is ethyl acetoacetate. Ethyl acetoacetate is an organic compound in which there are six carbon atoms, the second carbon atom has the ketone group and the fourth carbon atom has the ester functional group. The formula of Ethyl acetoacetate is given below:
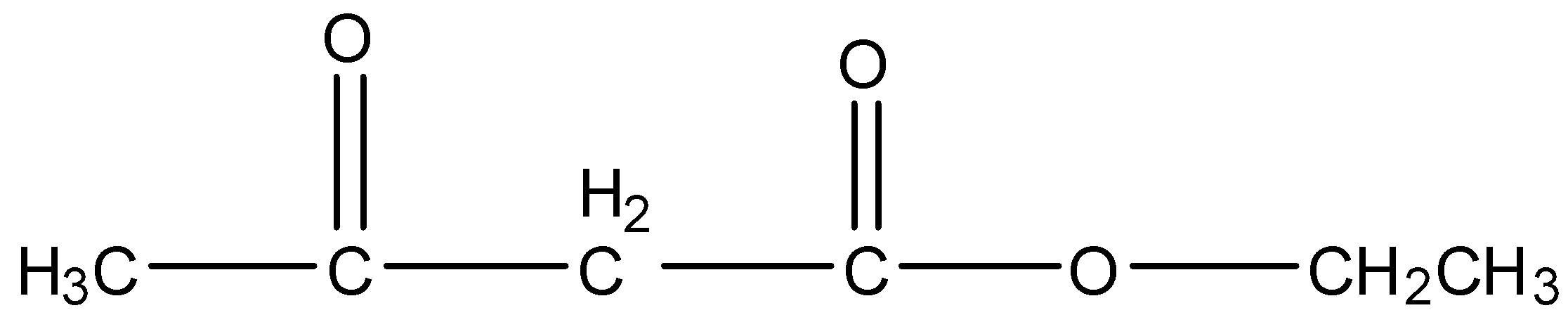
So, the third carbon atom in the compound, i.e., the methyl group between the ketone group and ester group is the most acidic group in the compound. Therefore, when the methyl magnesium iodide attacks the ethyl acetoacetate, then a hydrogen atom will be released from the third carbon atom of the ethyl acetoacetate. Since, after the release of the hydrogen atom, there will be a negative charge on the third carbon atom. In methyl magnesium iodide, magnesium iodide is the positive part and methyl group is the negative part. So, the positive part of the Grignard reagent will attack the negative part of the compound. The reaction is given below:
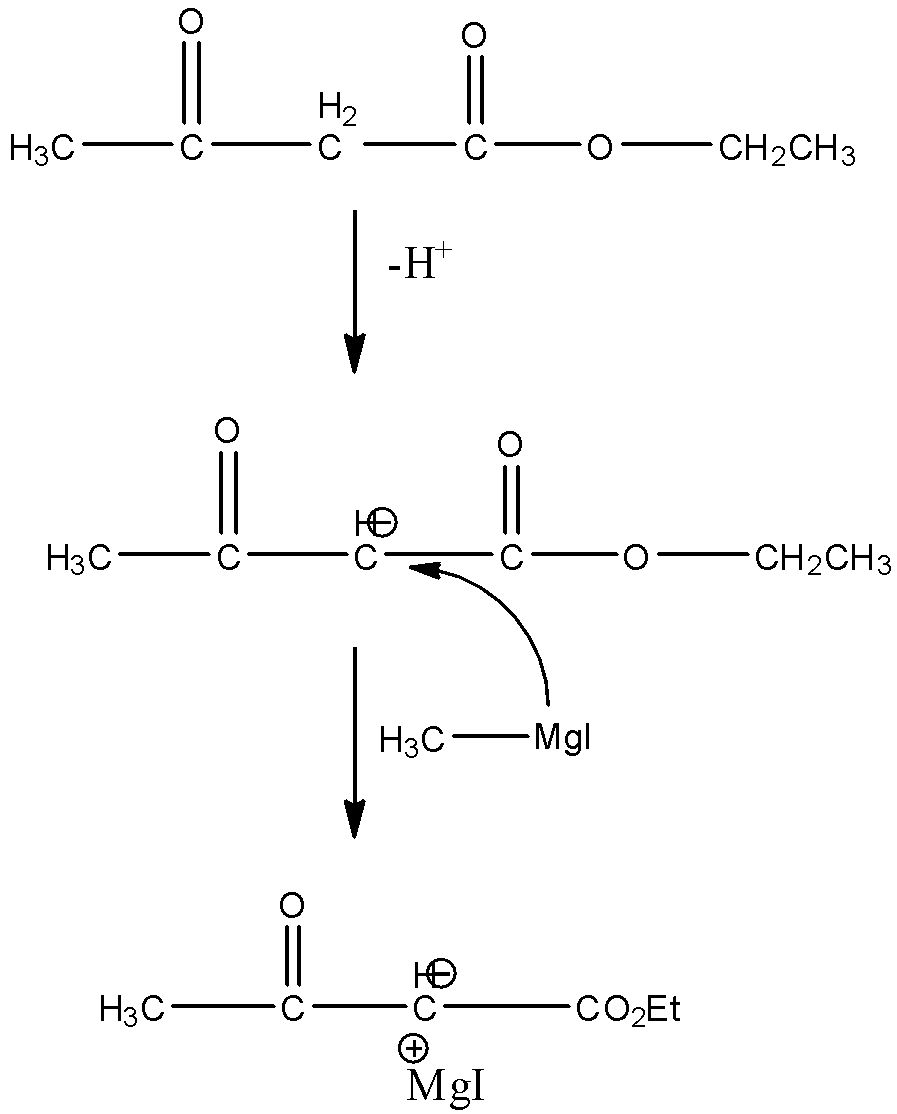
So, the correct answer is an option (c).
Note:
Don't get confused that, the Grignard reagent will attack the carbonyl group and will form alcohol, but we have to the most acidic part in the compound because the Grignard reagent attacks the acidic part of the compound.
Complete answer:
Methyl magnesium iodide is a Grignard reagent in which the halogen atom is iodine and an alkyl group is a methyl group. The formula of methyl magnesium iodide is $C{{H}_{3}}MgI$.
The given compound in the question is ethyl acetoacetate. Ethyl acetoacetate is an organic compound in which there are six carbon atoms, the second carbon atom has the ketone group and the fourth carbon atom has the ester functional group. The formula of Ethyl acetoacetate is given below:

So, the third carbon atom in the compound, i.e., the methyl group between the ketone group and ester group is the most acidic group in the compound. Therefore, when the methyl magnesium iodide attacks the ethyl acetoacetate, then a hydrogen atom will be released from the third carbon atom of the ethyl acetoacetate. Since, after the release of the hydrogen atom, there will be a negative charge on the third carbon atom. In methyl magnesium iodide, magnesium iodide is the positive part and methyl group is the negative part. So, the positive part of the Grignard reagent will attack the negative part of the compound. The reaction is given below:

So, the correct answer is an option (c).
Note:
Don't get confused that, the Grignard reagent will attack the carbonyl group and will form alcohol, but we have to the most acidic part in the compound because the Grignard reagent attacks the acidic part of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




