
How is ethanol prepared from?
1.Ethyl amine
2.Acetone
3.Formaldehyde
Answer
512.7k+ views
Hint: We have to know that ethanol is an alcohol containing two carbon atoms, one hydroxyl group and other hydrogen atoms. It can be commonly called ethyl alcohol, grain alcohol and grain alcohol. It is used in much organic synthesis and it is an industrial chemical. It is also used in beverages such as wine, alcohols.
Complete answer:
We will look at
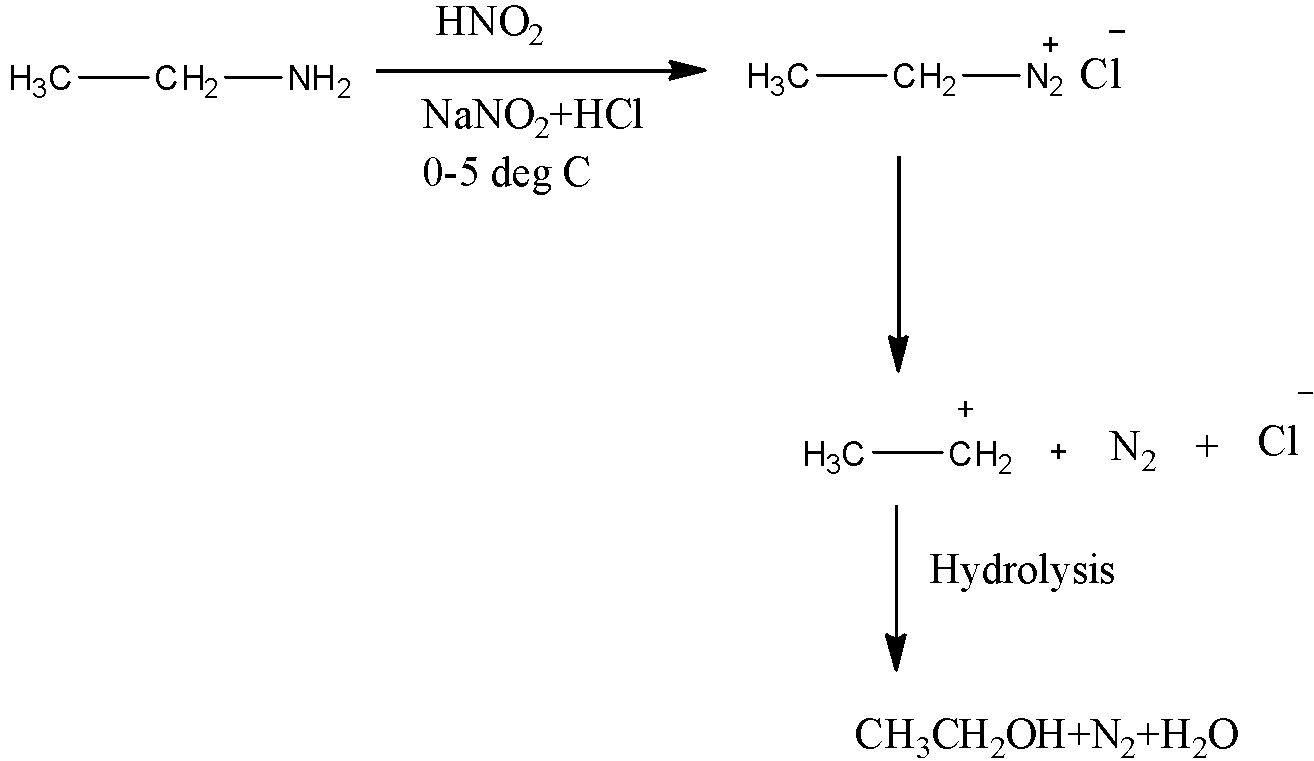
1. The preparation of ethanol from ethyl amine first. Ethyl amine is first treated with sodium nitrate and hydrochloric acid and nitric acid at low temperature, ethyl amine is then converted into its diazonium salt, it then dissociates into methyl ion, nitrogen and chloride ion which on hydrolysis gives ethanol, nitrogen gas and water.
2. Preparation of ethanol from acetone:
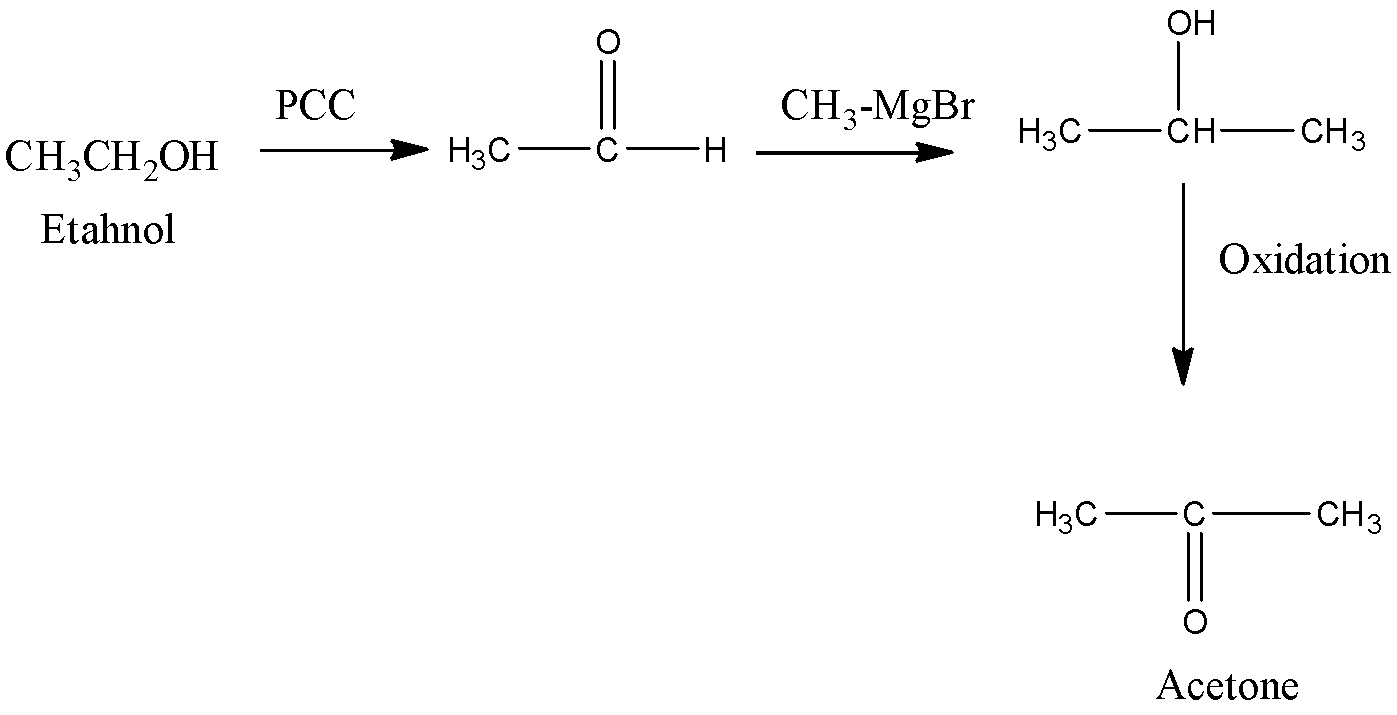
Ethanol is treated with pyridinium chlorochromate that works as a mild oxidizing agent. It converts alcohol into aldehyde thus ethanol is converted into ethanol. Then on further treating this aldehyde with Grignard reagent it gives Propan-1 –ol which on further oxidation
Preparation of ethanol from formaldehyde:
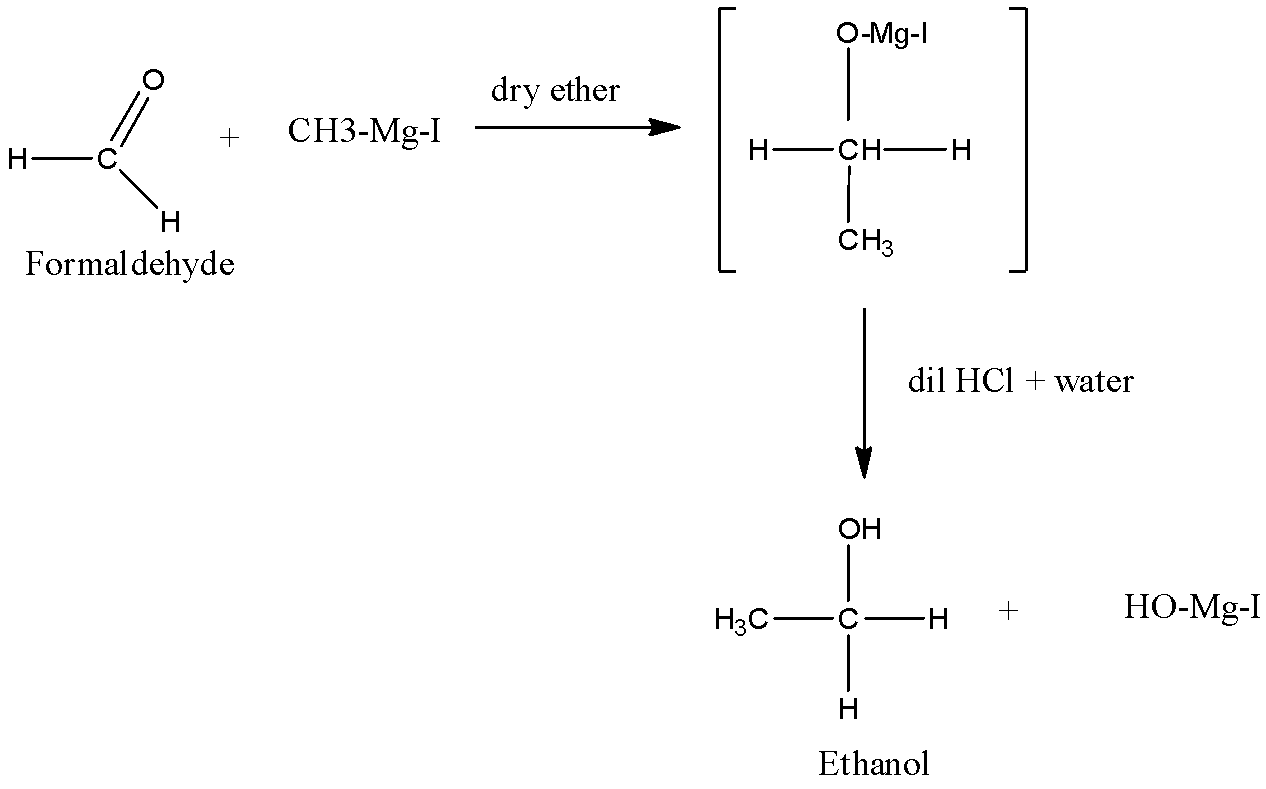
Formaldehyde is treated with Grignard reagent in presence of dry ether a complex is formed. On treating that complex with diluted hydrochloric acid and water, ethanol and magnesium iodide complex is formed. The reaction also goes further but we need the last product as ethanol thus the reaction steps are as followed.
Note:
We must have to remember thatEthanol is a colorless, volatile liquid. Formaldehyde is the simplest aldehyde containing only Hydrogen atoms and an aldehydic group. Acetone is the simplest ketone having a ketonic group in it. Pyridinium chlorochromate commonly known as PCC is used as an oxidizing agent.
Complete answer:
We will look at
1. The preparation of ethanol from ethyl amine first. Ethyl amine is first treated with sodium nitrate and hydrochloric acid and nitric acid at low temperature, ethyl amine is then converted into its diazonium salt, it then dissociates into methyl ion, nitrogen and chloride ion which on hydrolysis gives ethanol, nitrogen gas and water.

2. Preparation of ethanol from acetone:
Ethanol is treated with pyridinium chlorochromate that works as a mild oxidizing agent. It converts alcohol into aldehyde thus ethanol is converted into ethanol. Then on further treating this aldehyde with Grignard reagent it gives Propan-1 –ol which on further oxidation

Preparation of ethanol from formaldehyde:
Formaldehyde is treated with Grignard reagent in presence of dry ether a complex is formed. On treating that complex with diluted hydrochloric acid and water, ethanol and magnesium iodide complex is formed. The reaction also goes further but we need the last product as ethanol thus the reaction steps are as followed.

Note:
We must have to remember thatEthanol is a colorless, volatile liquid. Formaldehyde is the simplest aldehyde containing only Hydrogen atoms and an aldehydic group. Acetone is the simplest ketone having a ketonic group in it. Pyridinium chlorochromate commonly known as PCC is used as an oxidizing agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




