
When ethanol is treated with benzene diazonium chloride is forms:
A) Arenes
B) Methane
C) Amines
D) Ethyl alcohol
Answer
508.5k+ views
Hint: We have to know that the ethanol is considered to be a weak reducing agent, thus in this reaction also it reduces benzene diazonium chloride and itself gets reduced. Ethyl alcohol is commonly called ethanol and arenes are aromatic hydrocarbons as they involve ring structures and delocalization is present in the ring.
Complete answer:
We have to know that when ethanol is treated with benzene diazonium chloride it forms benzene and acetaldehyde as major products and hydrochloric acid and nitrogen gas is released. The chemical reaction can be represented as:
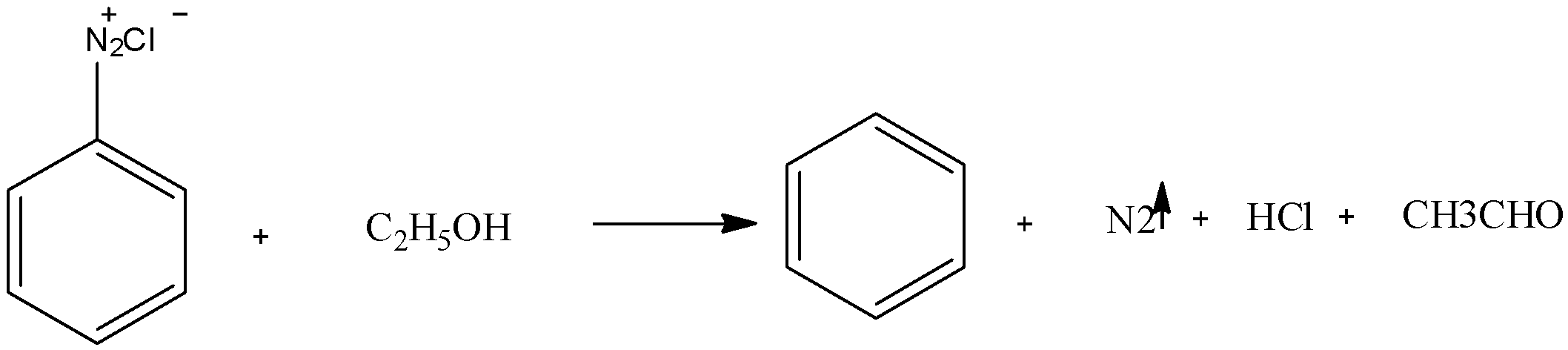
Benzene diazonium chloride salt can be formed by treating aniline in presence of sodium nitrate and hydrochloric acid, when benzene diazonium chloride is further treated with ethanol that is a mild reducing agent so it removes benzene from the diazonium salt and itself getting reduced to an aldehyde also producing nitrogen gas and hydrochloric acid as different products. Nitrogen gas is usually evolved in reaction involving benzene diazonium chloride as it has two nitrogen present in the structure thus dinitrogen can be easily escaped from the reaction.
When we look at all the option given;
Option A) is correct as arenes are aromatic hydrocarbons and simplest arene is benzene and we have shown in the chemical reaction that benzene is formed thus this is a correct option. Other options are not right as none of them is formed during chemical reaction.
Note:
We have to remember that this type of reaction can also be termed as deamination reaction. This reaction is a good method for converting benzene diazonium chloride into benzene, there are also many methods available but this is most suitable for getting benzene.
Complete answer:
We have to know that when ethanol is treated with benzene diazonium chloride it forms benzene and acetaldehyde as major products and hydrochloric acid and nitrogen gas is released. The chemical reaction can be represented as:

Benzene diazonium chloride salt can be formed by treating aniline in presence of sodium nitrate and hydrochloric acid, when benzene diazonium chloride is further treated with ethanol that is a mild reducing agent so it removes benzene from the diazonium salt and itself getting reduced to an aldehyde also producing nitrogen gas and hydrochloric acid as different products. Nitrogen gas is usually evolved in reaction involving benzene diazonium chloride as it has two nitrogen present in the structure thus dinitrogen can be easily escaped from the reaction.
When we look at all the option given;
Option A) is correct as arenes are aromatic hydrocarbons and simplest arene is benzene and we have shown in the chemical reaction that benzene is formed thus this is a correct option. Other options are not right as none of them is formed during chemical reaction.
Note:
We have to remember that this type of reaction can also be termed as deamination reaction. This reaction is a good method for converting benzene diazonium chloride into benzene, there are also many methods available but this is most suitable for getting benzene.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




