
Equivalent weight of $ M{n^{3 + }} $ in the following reaction is ( $ Mn = 55 $ ):
$ M{n^{3 + }}\xrightarrow{{}}M{n^{2 + }} + Mn{O_2} $
(A) $ 27.5 $
(B) $ 55 $
(C) $ 110 $
(D) $ 165 $
Answer
527.7k+ views
Hint: Here, a redox reaction is given and we have to calculate the equivalent weight of $ M{n^{3 + }} $ in the given reaction. So, first of all we have to understand the equivalent weight as it is the molar mass of a substance divided by the number of equivalents in the substance. Here, molar mass of the element $ Mn = 55 $ is given. Using this we can calculate the equivalent weight.
Complete answer:
Here, the disproportionate reaction is given in the question so we have to balance this reaction as below:
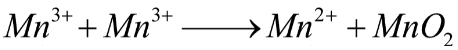
The half oxidation and reduction reactions are given below as:
$ {\text{oxidation reaction: }}M{n^{3 + }} + 2{H_2}O \to Mn{O_2} + 4{H^ + } + 1e $
$ {\text{reduction reaction: }}M{n^{3 + }} + 1e \to M{n^{2 + }} $
Now the molar mass of $ Mn $ is $ 55g{\left( {mole} \right)^{ - 1}} $
Let us use the formula for equivalent weight for half oxidation reaction as:
$ {\text{Equivalent weight of oxidising agent = }}\dfrac{{{\text{molar mass of }}Mn}}{{{\text{number of electron gained by one molecule}}}} $
Therefore,
$ {\text{Equivalent weight of oxidising agent = }}\dfrac{{55}}{1} = 55g.eq $
Now for other half reduction reaction the formula for equivalent weight is:
$ {\text{Equivalent weight of reducing agent = }}\dfrac{{{\text{molar mass of }}Mn{\text{ }}}}{{{\text{number of electrons lost by one molecule}}}} $
Therefore,
$ {\text{Equivalent weight of reducing agent = }}\dfrac{{55{\text{ }}}}{1} = 55g.eq $
So, the equivalent weight of $ M{n^{3 + }} $ is calculated as:
$ {\text{Equivalent weight of }}M{n^{3 + }} = {\text{Equivalent weight of oxidising agent}} + {\text{Equivalent weight of reducing agent}} $
$ \therefore {\text{Equivalent weight of }}M{n^{3 + }} = \left( {55 + 55} \right)g.eq $
$ \therefore {\text{Equivalent weight of }}M{n^{3 + }} = 110g.eq $
Thus, we have calculated the equivalent weight of the $ M{n^{3 + }} $ as $ 110g.eq $
Hence, the correct answer is option C.
Note:
Here, we have to understand the meaning of the equivalent weight as it is the molar mass of a substance divided by the number of equivalents in the substance and also that the reaction is redox as well as unbalanced. So we have to balance and also understand that we can be able to write it in half oxidation and half reduction reaction. We know that the gain of electrons represents oxidation and loss of electrons represents reduction reaction.
Complete answer:
Here, the disproportionate reaction is given in the question so we have to balance this reaction as below:

The half oxidation and reduction reactions are given below as:
$ {\text{oxidation reaction: }}M{n^{3 + }} + 2{H_2}O \to Mn{O_2} + 4{H^ + } + 1e $
$ {\text{reduction reaction: }}M{n^{3 + }} + 1e \to M{n^{2 + }} $
Now the molar mass of $ Mn $ is $ 55g{\left( {mole} \right)^{ - 1}} $
Let us use the formula for equivalent weight for half oxidation reaction as:
$ {\text{Equivalent weight of oxidising agent = }}\dfrac{{{\text{molar mass of }}Mn}}{{{\text{number of electron gained by one molecule}}}} $
Therefore,
$ {\text{Equivalent weight of oxidising agent = }}\dfrac{{55}}{1} = 55g.eq $
Now for other half reduction reaction the formula for equivalent weight is:
$ {\text{Equivalent weight of reducing agent = }}\dfrac{{{\text{molar mass of }}Mn{\text{ }}}}{{{\text{number of electrons lost by one molecule}}}} $
Therefore,
$ {\text{Equivalent weight of reducing agent = }}\dfrac{{55{\text{ }}}}{1} = 55g.eq $
So, the equivalent weight of $ M{n^{3 + }} $ is calculated as:
$ {\text{Equivalent weight of }}M{n^{3 + }} = {\text{Equivalent weight of oxidising agent}} + {\text{Equivalent weight of reducing agent}} $
$ \therefore {\text{Equivalent weight of }}M{n^{3 + }} = \left( {55 + 55} \right)g.eq $
$ \therefore {\text{Equivalent weight of }}M{n^{3 + }} = 110g.eq $
Thus, we have calculated the equivalent weight of the $ M{n^{3 + }} $ as $ 110g.eq $
Hence, the correct answer is option C.
Note:
Here, we have to understand the meaning of the equivalent weight as it is the molar mass of a substance divided by the number of equivalents in the substance and also that the reaction is redox as well as unbalanced. So we have to balance and also understand that we can be able to write it in half oxidation and half reduction reaction. We know that the gain of electrons represents oxidation and loss of electrons represents reduction reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




