
How many equivalent hydrogens are there in each isomer of ${{C}_{3}}{{H}_{7}}Cl$ ?
Answer
558.6k+ views
Hint: The hydrogens which are attached to a carbon with the same chemical environment are called equivalent hydrogens. Neopentane has 12 hydrogens and all 12 hydrogens are the same due to the same chemical environment.
Complete step by step answer:
- In the questions it is asked how many equivalent hydrogens are there in each isomer of ${{C}_{3}}{{H}_{7}}Cl$ .
- First we should know how many structural isomers are possible for ${{C}_{3}}{{H}_{7}}Cl$ .
- The structural isomers for the given compounds are two and they are 1 – Chloropropane and 2 – Chloropropane.
- To know about the number of equivalent hydrogens in 1 – Chloropropane and 2 – Chloropropane we should know the structures of those compounds.
- The structures of 1 – Chloropropane and 2 – Chloropropane are as follows.
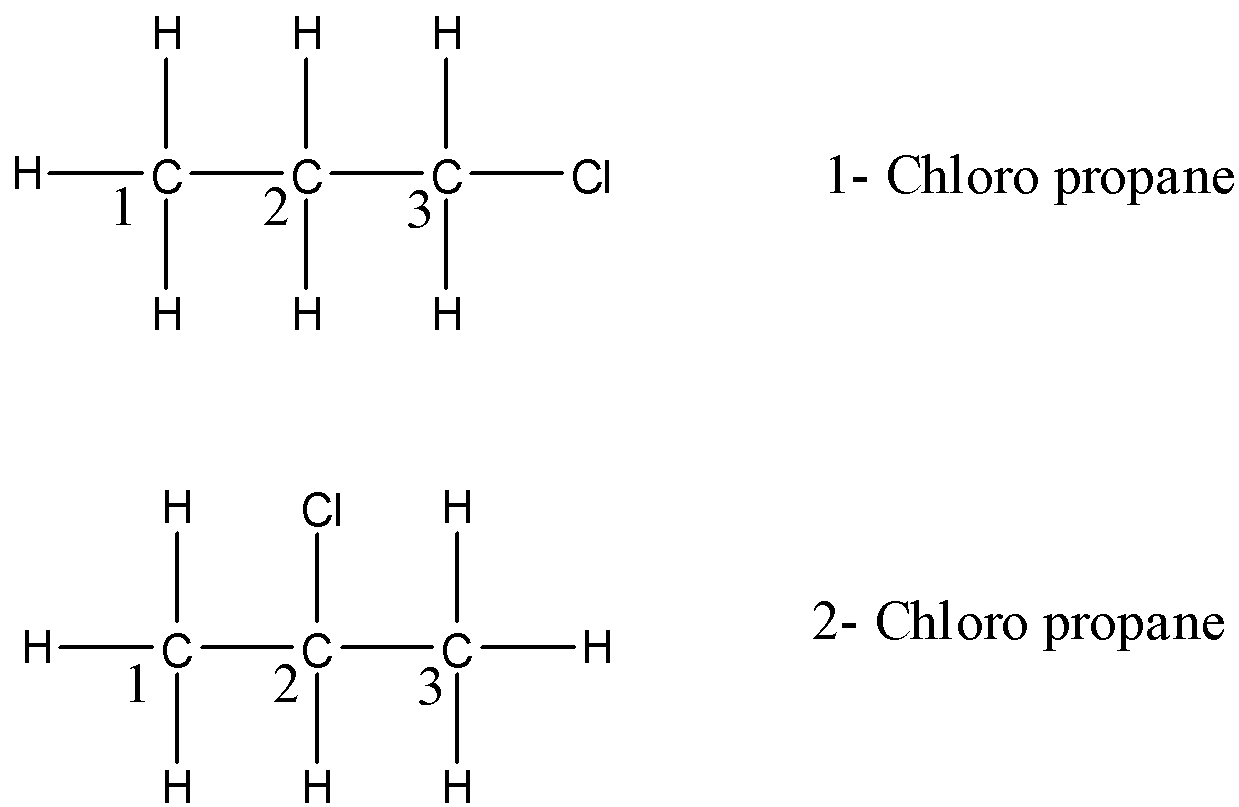
- From the above structures we can say that in 1 – Chloropropane there are three sets of equivalent protons present.
- In 1 – Chloropropane, on carbon-1 the three hydrogens have the same chemical environment and they are different from the hydrogen on carbon-2 because carbon-2 is nearer to chlorine which is present on carbon-1. So the hydrogens on carbon-1 are one set of equivalent protons.
- In 1 – Chloropropane, on carbon-2 the two hydrogens have the same chemical environment and they are different from the hydrogens on carbon-1 and carbon-3 because on carbon-1 there is a chlorine atom and on carbon-1 there is no chlorine. So, the hydrogens on carbon-2 are one set of equivalent hydrogens.
- In 1 – Chloropropane, on carbon-3 the two hydrogens have the same chemical environment and they are different from the hydrogens which are present on carbon-2 and carbon-3, because there is no chlorine on carbon-1 and carbon-3. So, the hydrogens on carbon-3 are one set of equivalent hydrogens.
- The same scenario repeats in the case of 2- chloro propane, it also has three sets of equivalent hydrogens.
- Therefore there are three sets of equivalent hydrogens present in each isomer of ${{C}_{3}}{{H}_{7}}Cl$ .
Note: By using proton NMR spectroscopy we can easily find the equivalent hydrogens, in the proton NMR spectrum of 1- chloropropane there are three peaks at three different positions that confirm the presence of three sets of equivalent hydrogens.
Complete step by step answer:
- In the questions it is asked how many equivalent hydrogens are there in each isomer of ${{C}_{3}}{{H}_{7}}Cl$ .
- First we should know how many structural isomers are possible for ${{C}_{3}}{{H}_{7}}Cl$ .
- The structural isomers for the given compounds are two and they are 1 – Chloropropane and 2 – Chloropropane.
- To know about the number of equivalent hydrogens in 1 – Chloropropane and 2 – Chloropropane we should know the structures of those compounds.
- The structures of 1 – Chloropropane and 2 – Chloropropane are as follows.

- From the above structures we can say that in 1 – Chloropropane there are three sets of equivalent protons present.
- In 1 – Chloropropane, on carbon-1 the three hydrogens have the same chemical environment and they are different from the hydrogen on carbon-2 because carbon-2 is nearer to chlorine which is present on carbon-1. So the hydrogens on carbon-1 are one set of equivalent protons.
- In 1 – Chloropropane, on carbon-2 the two hydrogens have the same chemical environment and they are different from the hydrogens on carbon-1 and carbon-3 because on carbon-1 there is a chlorine atom and on carbon-1 there is no chlorine. So, the hydrogens on carbon-2 are one set of equivalent hydrogens.
- In 1 – Chloropropane, on carbon-3 the two hydrogens have the same chemical environment and they are different from the hydrogens which are present on carbon-2 and carbon-3, because there is no chlorine on carbon-1 and carbon-3. So, the hydrogens on carbon-3 are one set of equivalent hydrogens.
- The same scenario repeats in the case of 2- chloro propane, it also has three sets of equivalent hydrogens.
- Therefore there are three sets of equivalent hydrogens present in each isomer of ${{C}_{3}}{{H}_{7}}Cl$ .
Note: By using proton NMR spectroscopy we can easily find the equivalent hydrogens, in the proton NMR spectrum of 1- chloropropane there are three peaks at three different positions that confirm the presence of three sets of equivalent hydrogens.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




