
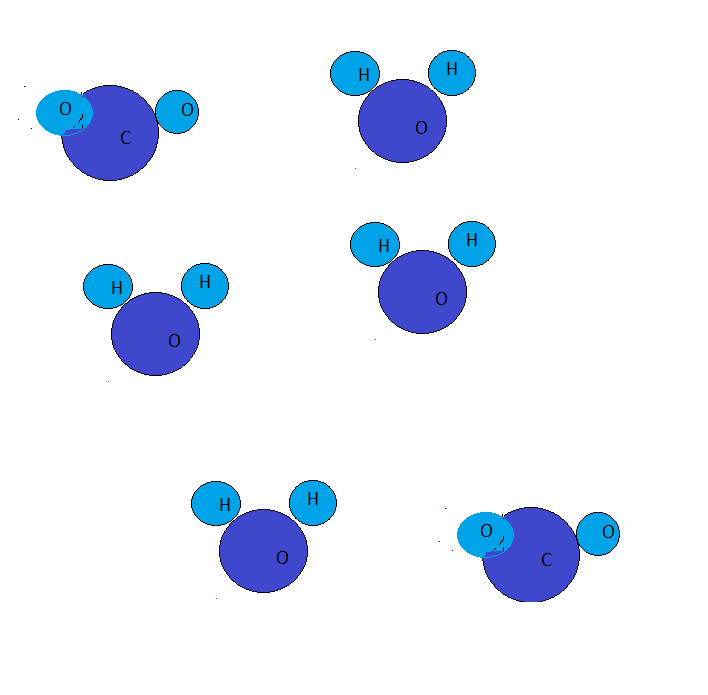
What is the empirical formula of the hydrocarbon \[\left( {{C_x}{H_y}} \right)\] that produced the mixture of products in the diagram after a combustion reaction?
A.\[C{H_4}\]
B.\[{C_2}{H_8}\]
C.\[{C_3}{H_4}\]
D.\[C{H_2}\]

Answer
497.4k+ views
Hint: Empirical formula is the shortest representation of molecular formula. All the atoms present in the molecular formula will be in the empirical formula. Alkanes are hydrocarbons when treated with oxygen forms carbon dioxide and water. These reactions are known as combustion reactions.
Complete answer:
Alkanes are saturated hydrocarbons consisting of only carbon and hydrogen atoms. When alkanes are treated with air in presence of oxygen gas leads to the formation of carbon dioxide along with water.
Given that an alkane undergoing combustion reaction produces six moles of water and two moles of carbon dioxide. Thus, the number of carbon atoms in the alkane are two and the number of hydrogen atoms are eight.
This leads to the result that alkane consists of four carbon atoms and eight hydrogen atoms.
Thus, the molecular formula of an alkane is \[{C_2}{H_8}\].
The empirical formula is the shortest representation of a molecular formula. The lowest ratio of atoms of the molecular formula can be written as \[C{H_4}\]
The empirical formula of a hydrocarbon \[\left( {{C_x}{H_y}} \right)\] that produced the mixture of products in the diagram after a combustion reaction is \[C{H_4}\].
Therefore, Option A is the correct one.
Note:
The combustion reaction is the reaction of alkanes with oxygen. All the atoms in alkane were formed as carbon dioxide and water. By looking at the products the molecular formula can be written. In the given options both molecular formula and empirical formula were there. But the empirical formula is the simplest ratio and it should be considered.
Complete answer:
Alkanes are saturated hydrocarbons consisting of only carbon and hydrogen atoms. When alkanes are treated with air in presence of oxygen gas leads to the formation of carbon dioxide along with water.
Given that an alkane undergoing combustion reaction produces six moles of water and two moles of carbon dioxide. Thus, the number of carbon atoms in the alkane are two and the number of hydrogen atoms are eight.
This leads to the result that alkane consists of four carbon atoms and eight hydrogen atoms.
Thus, the molecular formula of an alkane is \[{C_2}{H_8}\].
The empirical formula is the shortest representation of a molecular formula. The lowest ratio of atoms of the molecular formula can be written as \[C{H_4}\]
The empirical formula of a hydrocarbon \[\left( {{C_x}{H_y}} \right)\] that produced the mixture of products in the diagram after a combustion reaction is \[C{H_4}\].
Therefore, Option A is the correct one.
Note:
The combustion reaction is the reaction of alkanes with oxygen. All the atoms in alkane were formed as carbon dioxide and water. By looking at the products the molecular formula can be written. In the given options both molecular formula and empirical formula were there. But the empirical formula is the simplest ratio and it should be considered.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




