
How many elements are there on the Periodic Table $2020$ ?
Answer
510.3k+ views
Hint :Periodic table is also known as periodic table of elements. It is in a table form in which all the known (discovered) elements are arranged according to their atomic numbers, chemical properties and electronic configuration. The table has seven rows which are known as “periods” and eighteen columns which are known as “groups”.
Complete Step By Step Answer:
We know that, in a periodic table, all the known (discovered) elements have been arranged in the seven periods (rows) and eighteen (columns) of the table according to their atomic numbers, chemical properties and electronic configuration.
Now, we will see the period table:-
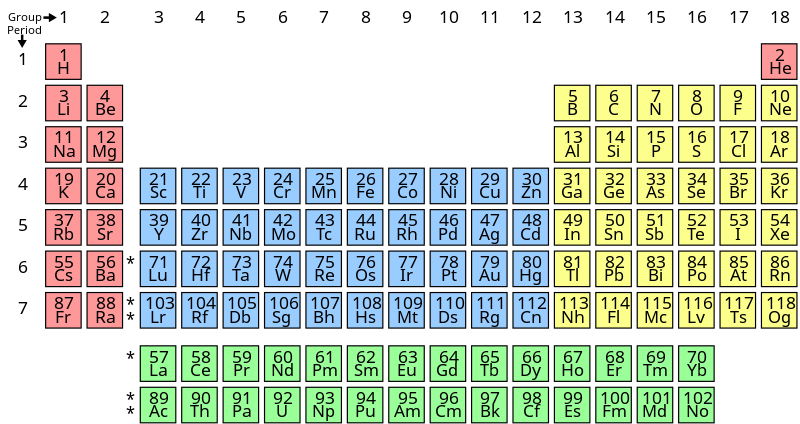
In the above periodic table, we can see that there are 118 known elements in 2020.
Additional Information:
The pink coloured elements in the group $1$ and group $2$ of the periodic table are known as s-block elements. They have general electronic configuration as $n{s^{1 - 2}}$ .
The elements in the blue coloured boxes from group $3$ to group $4$ are known as d-block elements or transition metals. Their general electronic configuration is $$(n - 1){d^{1 - 9}}n{s^{1 - 2}}$$ .
The elements in the yellow coloured boxes from group $13$ to group $18$ are known as p-block elements or main-group elements. Their general electronic configuration is $n{s^2}n{p^{1 - 6}}$ .
Group $18$elements are known as noble gases as they are very stable and inert in nature.
The elements in the green coloured boxes at the downward side of the periodic table are known as f-block elements. Their general electronic configuration is $(n - 2){f^{(0 - 14)}}(n - 1){d^{(0 - 10)}}n{s^2}$ . They are the members of group $3$ in the periodic table. They are also known as inner- transition metals.
Note :
F- block (actinides and lanthanides) elements comprise of $28$ elements and are always placed, with a notation, below the main body of the periodic table because actinides (Atomic numbers $58 - 71$ ) and lanthanides ( atomic numbers $90 - 103$ ) share similar chemical properties and cannot fit into the space inside the main body of periodic table.
Complete Step By Step Answer:
We know that, in a periodic table, all the known (discovered) elements have been arranged in the seven periods (rows) and eighteen (columns) of the table according to their atomic numbers, chemical properties and electronic configuration.
Now, we will see the period table:-

In the above periodic table, we can see that there are 118 known elements in 2020.
Additional Information:
The pink coloured elements in the group $1$ and group $2$ of the periodic table are known as s-block elements. They have general electronic configuration as $n{s^{1 - 2}}$ .
The elements in the blue coloured boxes from group $3$ to group $4$ are known as d-block elements or transition metals. Their general electronic configuration is $$(n - 1){d^{1 - 9}}n{s^{1 - 2}}$$ .
The elements in the yellow coloured boxes from group $13$ to group $18$ are known as p-block elements or main-group elements. Their general electronic configuration is $n{s^2}n{p^{1 - 6}}$ .
Group $18$elements are known as noble gases as they are very stable and inert in nature.
The elements in the green coloured boxes at the downward side of the periodic table are known as f-block elements. Their general electronic configuration is $(n - 2){f^{(0 - 14)}}(n - 1){d^{(0 - 10)}}n{s^2}$ . They are the members of group $3$ in the periodic table. They are also known as inner- transition metals.
Note :
F- block (actinides and lanthanides) elements comprise of $28$ elements and are always placed, with a notation, below the main body of the periodic table because actinides (Atomic numbers $58 - 71$ ) and lanthanides ( atomic numbers $90 - 103$ ) share similar chemical properties and cannot fit into the space inside the main body of periodic table.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




