
What is the electron geometry and molecular geometry of \[{H_2}O\] ?
Answer
478.8k+ views
Hint: The recognition of a bent molecule is easy if the molecule has any lone pair of electrons. In the case of water, even though it has bonds that are related to the H, it additionally has lone pairs which push down on the \[2\]bonds and make a "bent" form in preference to it being linear.
Complete answer:
The digital geometry offers water a tetrahedral form.
The molecular geometry offers water a dishonest form.
Electronic geometry takes into consideration the electron pairs that aren't taking part in bonding and the electron cloud density.
Here the two bonds of hydrogen matter as\[2\]electron clouds and the two-electron pairs matter as some other \[2\] giving us a complete of four. With four electron regions, the VSEPR digital geometry is tetrahedral.
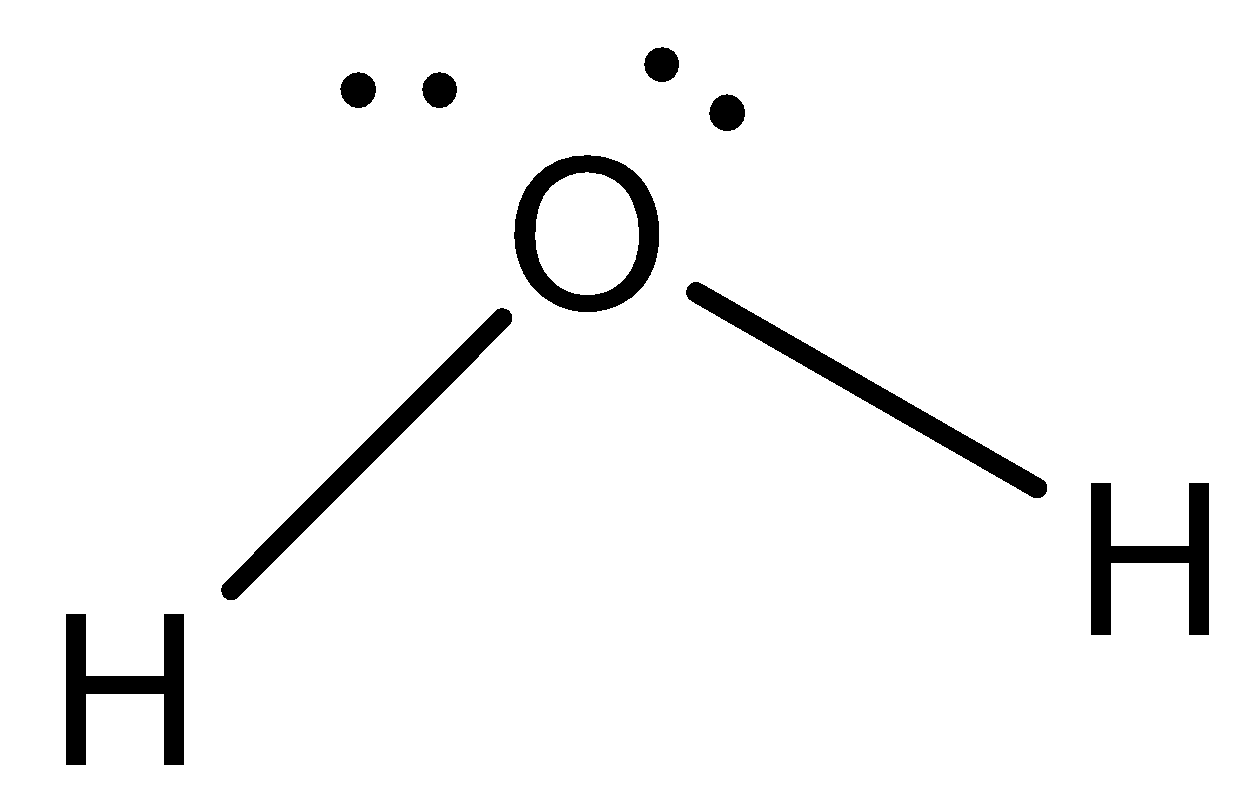
Molecular geometry seems at best the one’s electrons that are taking part in bonding. So here, best the two bonds to H are taken into consideration.
The form might now no longer be linear, as in the case with \[C{O_2}\], despite the fact that there are best \[2\]bonds in \[{H_2}O\] because of the truth that the unpaired electrons might now no longer permit this type of form. There is electron repulsion occurring so electrons tackle shapes that assist to lessen electron repulsion.
Note:
However, in liquid water or ice, the lone pairs shape hydrogen bonds with neighboring water molecules. The maximum not unusual place association of hydrogen atoms round oxygen is tetrahedral with hydrogen atoms covalently bonded to oxygen and connected via way of means of hydrogen bonds. Since the hydrogen bonds range in duration a lot of those water molecules aren't symmetrical and shape brief abnormal tetrahedral among their \[4\]related hydrogen atoms.
Complete answer:
The digital geometry offers water a tetrahedral form.
The molecular geometry offers water a dishonest form.
Electronic geometry takes into consideration the electron pairs that aren't taking part in bonding and the electron cloud density.
Here the two bonds of hydrogen matter as\[2\]electron clouds and the two-electron pairs matter as some other \[2\] giving us a complete of four. With four electron regions, the VSEPR digital geometry is tetrahedral.

Molecular geometry seems at best the one’s electrons that are taking part in bonding. So here, best the two bonds to H are taken into consideration.
The form might now no longer be linear, as in the case with \[C{O_2}\], despite the fact that there are best \[2\]bonds in \[{H_2}O\] because of the truth that the unpaired electrons might now no longer permit this type of form. There is electron repulsion occurring so electrons tackle shapes that assist to lessen electron repulsion.
Note:
However, in liquid water or ice, the lone pairs shape hydrogen bonds with neighboring water molecules. The maximum not unusual place association of hydrogen atoms round oxygen is tetrahedral with hydrogen atoms covalently bonded to oxygen and connected via way of means of hydrogen bonds. Since the hydrogen bonds range in duration a lot of those water molecules aren't symmetrical and shape brief abnormal tetrahedral among their \[4\]related hydrogen atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




