
What is the electron configuration for cobalt z = 27? Why?
Answer
524.1k+ views
Hint: The electronic configuration of any element tells us the total number of electrons present in that atom. It is identified through atomic number, as the atomic number of an atom tells us the total number of electrons in that atom. The electronic configuration requires filling of orbital or sub shells (s, p, d, f) according to Aufbau principle.
Complete answer:
The Electronic configuration of any atom tells us the total number of electrons which is equal to the total number protons in that atom that is equal to the atomic number of that element. Electronic configuration of any element consists of filling the orbital or the s, p, d, f sub – shells with electrons. The filling of electrons in various orbits is according to a principle of Aufbau that takes place from the lower energy level to the higher energy level. The s, p, d, f subshells are written along with the number of the shell, like 1,2, 3, etc.
We have been given cobalt which has atomic number z = 27. The electronic configuration of cobalt will consist of filling 27 electrons according to the Aufbau principle. The Aufbau diagram that shows filling is,
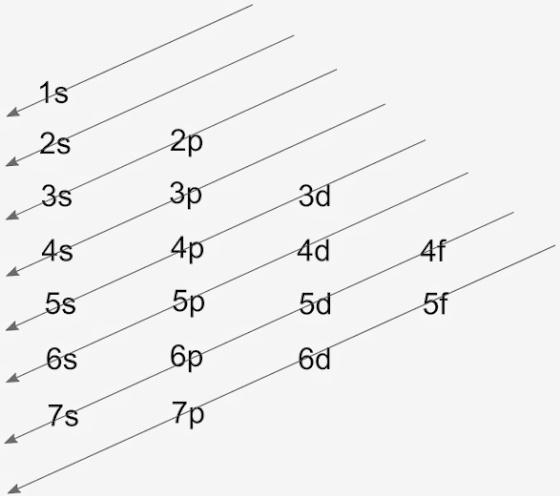
The arrows denote the manner of filling. So, through this diagram the configuration of cobalt is, $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{7}}$
Hence, the electronic configuration of cobalt is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{7}}$that has the energy levels and subshells occupied by the electrons according to the Aufbau principle.
Note:
Another type of configuration called as shorthand or noble gas configuration is used to write the configuration of elements that ease them in representing the atoms. The shorthand configuration of cobalt is $[Ar]4{{s}^{2}}3{{d}^{7}}$, where argon is noble gas with z = 18. Cobalt is the element of the d block, so the valence shell in this atom enters into the d – orbital.
Complete answer:
The Electronic configuration of any atom tells us the total number of electrons which is equal to the total number protons in that atom that is equal to the atomic number of that element. Electronic configuration of any element consists of filling the orbital or the s, p, d, f sub – shells with electrons. The filling of electrons in various orbits is according to a principle of Aufbau that takes place from the lower energy level to the higher energy level. The s, p, d, f subshells are written along with the number of the shell, like 1,2, 3, etc.
We have been given cobalt which has atomic number z = 27. The electronic configuration of cobalt will consist of filling 27 electrons according to the Aufbau principle. The Aufbau diagram that shows filling is,

The arrows denote the manner of filling. So, through this diagram the configuration of cobalt is, $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{7}}$
Hence, the electronic configuration of cobalt is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{7}}$that has the energy levels and subshells occupied by the electrons according to the Aufbau principle.
Note:
Another type of configuration called as shorthand or noble gas configuration is used to write the configuration of elements that ease them in representing the atoms. The shorthand configuration of cobalt is $[Ar]4{{s}^{2}}3{{d}^{7}}$, where argon is noble gas with z = 18. Cobalt is the element of the d block, so the valence shell in this atom enters into the d – orbital.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




