
During osmosis, the flow of water through a semipermeable membrane is:
(A) From a solution having higher concentration only
(B) Form both sides of the semipermeable membrane with equal flow rates
(C) From both sides of the semipermeable membrane with unequal flow rates
(D) From a solution having lower concentration only
Answer
586.2k+ views
Hint: The osmosis is a process of movement of solute from the higher concentrated solution to the lower concentrated solution. The semi-permeable membrane (SPM) separates the solution in such a way that the solvent is allowed to move across. The pressure associated with the no movement of the solute and solvent is known as osmotic pressure. It is a colligative property.
Complete step by step answer: There is a natural tendency of the solutes in the solution to move or diffuse from a higher concentration to the lower concentration to bring a uniform distribution of solute throughout the solution. This is called osmosis.
For example, if we place a concentrated solution of copper sulphate at the bottom of the beaker and a layer of water, or that the dilute solution of copper sulphate is carefully poured over it, there will be a distinct or well-defined boundary between the layers. After some time, the boundary will dissolve or become indistinct since the solute is distributed uniformly and forms a homogeneous solution.
Let's consider a solution that is separated from the pure solvent by the semi-permeable membrane (SPM). That is a membrane that allows the free movement of solvent molecules only but not the solute, molecules across it.
The semipermeable membrane has a special feature. The pore size of the membrane is small that allows only the solvent molecules to pass through it. It only allows their movement. However, the solute particles are considered to have a bigger size than the solvent molecules, thus not allowing them to move across the membrane.
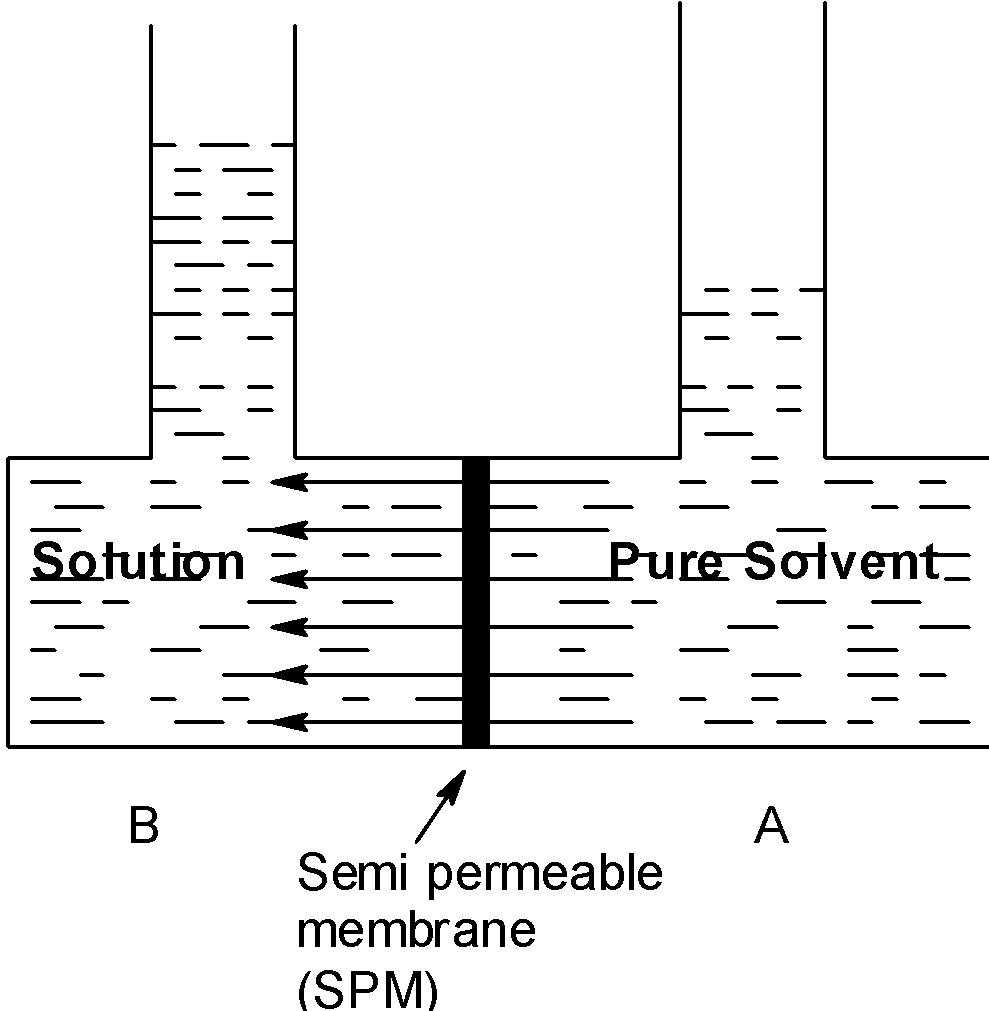
The SPM separates the solution from the pure solvent. The solution contains the solute particles thus the concentration of solvent is less in the solution however, the pure solvent is at the higher concentrations.
Therefore, the solvent molecules move from the pure solvent side to the solution side, where the concentration of the solvent is low.
Thus, during the osmosis, the flow of water is from high concentration solutions only.
So, the correct answer is “Option A”.
Note: There is a process of movement of solute particles from the more concentrated to the less concentrated solution. It is noted as the diffusion. Students should remember that osmosis is also known as diffusion. The SPM is selective towards solvent only and has restrictions for solute particles. It is possible to prevent the flow of solvent through the SPM by applying sufficient pressure on the solution called the osmotic pressure.
Complete step by step answer: There is a natural tendency of the solutes in the solution to move or diffuse from a higher concentration to the lower concentration to bring a uniform distribution of solute throughout the solution. This is called osmosis.
For example, if we place a concentrated solution of copper sulphate at the bottom of the beaker and a layer of water, or that the dilute solution of copper sulphate is carefully poured over it, there will be a distinct or well-defined boundary between the layers. After some time, the boundary will dissolve or become indistinct since the solute is distributed uniformly and forms a homogeneous solution.
Let's consider a solution that is separated from the pure solvent by the semi-permeable membrane (SPM). That is a membrane that allows the free movement of solvent molecules only but not the solute, molecules across it.
The semipermeable membrane has a special feature. The pore size of the membrane is small that allows only the solvent molecules to pass through it. It only allows their movement. However, the solute particles are considered to have a bigger size than the solvent molecules, thus not allowing them to move across the membrane.

The SPM separates the solution from the pure solvent. The solution contains the solute particles thus the concentration of solvent is less in the solution however, the pure solvent is at the higher concentrations.
Therefore, the solvent molecules move from the pure solvent side to the solution side, where the concentration of the solvent is low.
Thus, during the osmosis, the flow of water is from high concentration solutions only.
So, the correct answer is “Option A”.
Note: There is a process of movement of solute particles from the more concentrated to the less concentrated solution. It is noted as the diffusion. Students should remember that osmosis is also known as diffusion. The SPM is selective towards solvent only and has restrictions for solute particles. It is possible to prevent the flow of solvent through the SPM by applying sufficient pressure on the solution called the osmotic pressure.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




