
During fractional distillation, the crude petroleum is heated to a temperature of about
A. $ {600^o}C $
B. $ 400 - {500^o}C $
C. $ {200^o}C $
D. $ {100^o}C $
Answer
494.4k+ views
Hint: The process of separating a substance into its fractions or parts by taking advantage of different vapour pressure of those substances is known as Fractional distillation. It is frequently used as a synonym with distillation as it refers to the separation of components in a mixture having difference in their boiling point.
Complete answer:
In fractional distillation, a long fractionating column is fitted above the mixture in which several condensers are placed at different heights. The bottom part of this fractionating column is taken hot while it is cool at the top. Components with high boiling point tend to condense at the bottom while the substances with lower boiling points condense at the top of the fractionating column.
We know that crude petroleum or oil is a mixture of several molecules of hydrocarbons which is evaporated and its vapours condense at different temperatures in the fractionating column. Each fraction consists of molecules of hydrocarbon with the same number of carbon atoms and with a similar range of boiling points. Condensation of different hydrocarbons at certain range of temperature is shown as follows:
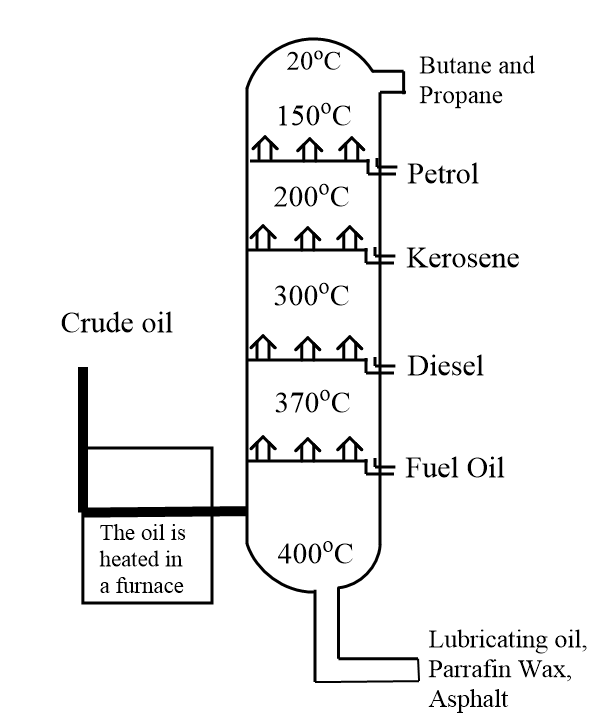
Hence, during fractional distillation crude oil or petroleum is heated to a temperature of about $ 400 - {500^o}C $ which allows most of the oil to evaporate and enter the column.
Thus, option (B) is the correct answer.
Note:
It is important to note that the temperature at the bottom is always considered higher so that the hydrocarbons with longer carbon chains can fall out at the bottom and the shorter carbon chains will go upwards in the column until they hit a temperature at which they can be condensed.
Complete answer:
In fractional distillation, a long fractionating column is fitted above the mixture in which several condensers are placed at different heights. The bottom part of this fractionating column is taken hot while it is cool at the top. Components with high boiling point tend to condense at the bottom while the substances with lower boiling points condense at the top of the fractionating column.
We know that crude petroleum or oil is a mixture of several molecules of hydrocarbons which is evaporated and its vapours condense at different temperatures in the fractionating column. Each fraction consists of molecules of hydrocarbon with the same number of carbon atoms and with a similar range of boiling points. Condensation of different hydrocarbons at certain range of temperature is shown as follows:

Hence, during fractional distillation crude oil or petroleum is heated to a temperature of about $ 400 - {500^o}C $ which allows most of the oil to evaporate and enter the column.
Thus, option (B) is the correct answer.
Note:
It is important to note that the temperature at the bottom is always considered higher so that the hydrocarbons with longer carbon chains can fall out at the bottom and the shorter carbon chains will go upwards in the column until they hit a temperature at which they can be condensed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




