
Draw the structures of the following molecules:
(i) \[{\text{Xe}}{{\text{F}}_{\text{6}}}\]
(ii) \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\]
Answer
569.4k+ views
Hint:(i) First write the chemical formula of the cation present. Then, find out the steric number of the central xenon atom in the cation. Then from the steric number, find out the type of hybridisation.
(ii) Compare the structure of \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] with the structure of sulphuric acid.
Complete answer:
(i) The chemical formula \[{\text{Xe}}{{\text{F}}_{\text{6}}}\] represents xenon hexafluoride.
he atomic number of xenon is 54 and its electronic configuration is \[\left[ {{\text{Kr}}} \right]4{d^{10}}5{s^2}5{p^6}\]
In the outermost shell (the valence shell), the xenon atom has 8 electrons.
Xenon atoms form six bonds with six fluorine atoms by sharing its six electrons. A Xenon atom has six bond pairs of electrons and one lone pair of electrons. The steric number of xenon in the cation \[{\text{Xe}}{{\text{F}}_{\text{6}}}\] is 7.
The type of hybridization associated with the steric number of 6 is \[s{p^3}{d^3}\].
The molecular geometry is distorted octahedral. Write the structure of \[{\text{Xe}}{{\text{F}}_{\text{6}}}\] as shown below.
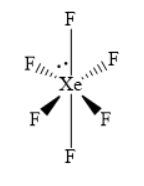
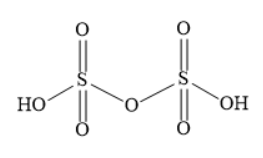
(ii) The molecule \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] is an anhydride of sulphuric acid. In the structure of \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] two tetrahedral units share one corner. In each tetrahedral unit, the central sulphur atom forms two double bonds with two oxygen atoms and the sulphur atom also forms two single bonds with two other oxygen atoms. In each tetrahedral unit, the oxygen atoms are present at four corners of a regular tetrahedron.
Write the structure of \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] as shown below:
Note:
The steric number is the total number of bond pairs and lone pairs of electrons present around the central atom in the molecule / ion. Thus, if 6 bond pairs and 1 lone pair of electrons are present, the steric number is \[6 + 1 = 7\] .
(ii) Compare the structure of \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] with the structure of sulphuric acid.
Complete answer:
(i) The chemical formula \[{\text{Xe}}{{\text{F}}_{\text{6}}}\] represents xenon hexafluoride.
he atomic number of xenon is 54 and its electronic configuration is \[\left[ {{\text{Kr}}} \right]4{d^{10}}5{s^2}5{p^6}\]
In the outermost shell (the valence shell), the xenon atom has 8 electrons.
Xenon atoms form six bonds with six fluorine atoms by sharing its six electrons. A Xenon atom has six bond pairs of electrons and one lone pair of electrons. The steric number of xenon in the cation \[{\text{Xe}}{{\text{F}}_{\text{6}}}\] is 7.
The type of hybridization associated with the steric number of 6 is \[s{p^3}{d^3}\].
The molecular geometry is distorted octahedral. Write the structure of \[{\text{Xe}}{{\text{F}}_{\text{6}}}\] as shown below.

(ii) The molecule \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] is an anhydride of sulphuric acid. In the structure of \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] two tetrahedral units share one corner. In each tetrahedral unit, the central sulphur atom forms two double bonds with two oxygen atoms and the sulphur atom also forms two single bonds with two other oxygen atoms. In each tetrahedral unit, the oxygen atoms are present at four corners of a regular tetrahedron.
Write the structure of \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{7}}}\] as shown below:

Note:
The steric number is the total number of bond pairs and lone pairs of electrons present around the central atom in the molecule / ion. Thus, if 6 bond pairs and 1 lone pair of electrons are present, the steric number is \[6 + 1 = 7\] .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




