
Draw the structures of the following molecules:
A.${{H}_{3}}P{{O}_{2}}$
B.$Cl{{F}_{3}}$
Answer
531.6k+ views
Hint: In this question, we have to draw the structures of ${{H}_{3}}P{{O}_{2}}$and $Cl{{F}_{3}}$. ${{H}_{3}}P{{O}_{2}}$ which is known as Hypophosphorous acid. It is a phosphorus oxyacid which is a strong reducing agent. It is also called phosphinic acid. $Cl{{F}_{3}}$is chlorine trifluoride which is formed by fluorination of chlorine.
Complete answer:
${{H}_{3}}P{{O}_{2}}$- It is a colorless compound that is water-soluble. It’s a low-melting compound. It is also soluble in dioxane and alcohol. The more descriptive representation of it is $HOP(O){{H}_{2}}$. It has a monoprotic character. Hypophosphites are the name of the salts that are derived from this acid.
$HOP(O){{H}_{2}}$ occurs in the equilibrium with slight tautomer $HP{{(OH)}_{2}}$, It is called hypophosphorous acid whereas the major tautomer is phosphonic acid.
Preparation-
The white phosphorus reacts with a hot aqueous solution of a hydroxide results in the formation of hypophosphite salts.
${{P}_{4}}+O{{H}^{-}}+4{{H}_{2}}O\to 4{{H}_{2}}P{{O}_{2}}^{-}+2{{H}_{2}}$
A strong non-oxidizing acid is reacted with the salt to give hypophosphorous acid.
${{H}_{2}}P{{O}_{2}}^{-}+{{H}^{+}}\to {{H}_{3}}P{{O}_{2}}$
${{H}_{3}}P{{O}_{2}}$is used as a 50% aqueous solution. The evaporation of water cannot form anhydrous acid because the acid disproportionates to phosphorus and phosphine, it gets oxidized to phosphorous acid and phosphoric acid. The continuous extraction of aqueous solution with diethyl ether forms pure anhydrous hypophosphorous acid.
Structure of ${{H}_{3}}P{{O}_{2}}$ is :
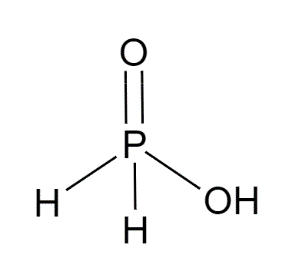
The molecular shape of ${{H}_{3}}P{{O}_{2}}$is pseudo-tetrahedral.
$Cl{{F}_{3}}$- In 1930, Ruff and Krug prepared it by fluorination of chlorine, It also gave ClF, and Distillation was used to separate the mixture.
Preparation-
$3{{F}_{2}}+C{{l}_{2}}\to 2Cl{{F}_{3}}$
Structure of $Cl{{F}_{3}}$ is :

The molecular geometry of $Cl{{F}_{3}}$ is T-shaped.
The structure was predicted by VSEPR theory.
Note:
Uses of ${{H}_{3}}P{{O}_{2}}$ are- It is utilized as a salt (Sodium hypophosphite). It is also used in the electroless plating of Nickel.
Uses of $Cl{{F}_{3}}$are- It helps in cleaning of the chemical vapor deposition chambers in the semiconductor industry. It can be used as a storable oxidizer in rocket propellant systems.
Complete answer:
${{H}_{3}}P{{O}_{2}}$- It is a colorless compound that is water-soluble. It’s a low-melting compound. It is also soluble in dioxane and alcohol. The more descriptive representation of it is $HOP(O){{H}_{2}}$. It has a monoprotic character. Hypophosphites are the name of the salts that are derived from this acid.
$HOP(O){{H}_{2}}$ occurs in the equilibrium with slight tautomer $HP{{(OH)}_{2}}$, It is called hypophosphorous acid whereas the major tautomer is phosphonic acid.
Preparation-
The white phosphorus reacts with a hot aqueous solution of a hydroxide results in the formation of hypophosphite salts.
${{P}_{4}}+O{{H}^{-}}+4{{H}_{2}}O\to 4{{H}_{2}}P{{O}_{2}}^{-}+2{{H}_{2}}$
A strong non-oxidizing acid is reacted with the salt to give hypophosphorous acid.
${{H}_{2}}P{{O}_{2}}^{-}+{{H}^{+}}\to {{H}_{3}}P{{O}_{2}}$
${{H}_{3}}P{{O}_{2}}$is used as a 50% aqueous solution. The evaporation of water cannot form anhydrous acid because the acid disproportionates to phosphorus and phosphine, it gets oxidized to phosphorous acid and phosphoric acid. The continuous extraction of aqueous solution with diethyl ether forms pure anhydrous hypophosphorous acid.
Structure of ${{H}_{3}}P{{O}_{2}}$ is :

The molecular shape of ${{H}_{3}}P{{O}_{2}}$is pseudo-tetrahedral.
$Cl{{F}_{3}}$- In 1930, Ruff and Krug prepared it by fluorination of chlorine, It also gave ClF, and Distillation was used to separate the mixture.
Preparation-
$3{{F}_{2}}+C{{l}_{2}}\to 2Cl{{F}_{3}}$
Structure of $Cl{{F}_{3}}$ is :

The molecular geometry of $Cl{{F}_{3}}$ is T-shaped.
The structure was predicted by VSEPR theory.
Note:
Uses of ${{H}_{3}}P{{O}_{2}}$ are- It is utilized as a salt (Sodium hypophosphite). It is also used in the electroless plating of Nickel.
Uses of $Cl{{F}_{3}}$are- It helps in cleaning of the chemical vapor deposition chambers in the semiconductor industry. It can be used as a storable oxidizer in rocket propellant systems.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




